Cold-shock eliminates female nucleus in fertilized eggs to induce androgenesis in the loach (Misgurnus anguillicaudatus), a teleost fish
- PMID: 22122997
- PMCID: PMC3266226
- DOI: 10.1186/1472-6750-11-116
Cold-shock eliminates female nucleus in fertilized eggs to induce androgenesis in the loach (Misgurnus anguillicaudatus), a teleost fish
Abstract
Background: Androgenesis (all-male inheritance) is generally induced by means of irradiating the eggs to inactivate the maternal genome, followed by fertilization with normal sperm. In fish, the conventional technique for induced androgenesis has been applied for rapid fixation to traits, recovery of cryopreserved genotypes, sex-control, etc. A new method of androgenesis that eliminates the need to irradiate the egg was proposed using the loach, Misgurnus anguillicaudatus (a teleost fish).
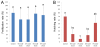
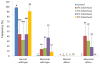
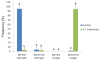
Results: When the eggs of wild-type females were fertilized with sperm of albino or orange phenotype males and cold-shocked at 0 to 3°C for 60 min duration just after fertilization, generally more than 30% (with a peak of 100%) of the hatched progeny were androgenotes. While a few of them were the normal diploid, most of them turned out to be abnormal haploid. All-male inheritance was verified by the expression of the recessive color trait (albino or orange) and microsatellite genotypes comprising only paternally derived alleles. Nuclear behavior after the cold-shock treatment was traced by microscopic observation of DAPI (4'6-diamidino-2-phenylindole)-stained samples and hematoxylin-eosin stained histological sections, and the extrusion of egg (maternal) nucleus was observed in eggs treated in the optimum timing.
Conclusion: In this paper, we demonstrate that cold-shock treatment (at 0 and 3°C) of loach eggs for 60 min just after fertilization successfully induces androgenetic haploid development. The most likely mechanism of cold-shock induced androgenesis is an elimination of the egg nucleus together along with the second polar body and subsequent development of a decondensed sperm nucleus or male pronucleus.
Figures









References
-
- Pandian TJ, Koteeswaran R. Ploidy induction and sex control in fish. Hydrobiologia. 1998;384:167–243. doi: 10.1023/A:1003332526659. - DOI
-
- Komen H, Thorgaard GH. Androgenesis, gynogenesis and the production of clones in fishes: a review. Aquaculture. 2007;269:150–173. doi: 10.1016/j.aquaculture.2007.05.009. - DOI
-
- Fujimoto T, Sakao S, Yamaha E, Arai K. Evaluation of different doses of UV irradiation to loach eggs for genetic inactivation of the maternal genome. J Exp Zool Part A Ecol Genet Physiol. 2007;307:449–462. - PubMed
-
- Sakao S, Fujimoto T, Kimura S, Yamaha E, Arai K. Drastic mortality in tetraploid induction results from the elevation of ploidy in masu salmon Oncorhynchus masou. Aquaculture. 2006;252:147–160. doi: 10.1016/j.aquaculture.2005.06.048. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

