Smooth muscle α actin is specifically required for the maintenance of lactation
- PMID: 22123032
- PMCID: PMC4151467
- DOI: 10.1016/j.ydbio.2011.11.002
Smooth muscle α actin is specifically required for the maintenance of lactation
Abstract
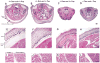
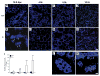
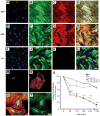
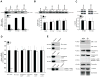
Smooth muscle α-actin (Acta2) is one of six highly conserved mammalian actin isoforms that appear to exhibit functional redundancy. Nonetheless, we have postulated a specific functional role for the smooth muscle specific isoform. Here, we show that Acta2 deficient mice have a remarkable mammary phenotype such that dams lacking Acta2 are unable to nurse their offspring effectively. The phenotype was rescued in cross fostering experiments with wild type mice, excluding a developmental defect in Acta2 null pups. The mechanism for the underlying phenotype is due to myoepithelial dysfunction postpartum resulting in precocious involution. Further, we demonstrate a specific defect in myoepithelial cell contractility in Acta2 null mammary glands, despite normal expression of cytoplasmic actins. We conclude that Acta2 specifically mediates myoepithelial cell contraction during lactation and that this actin isoform therefore exhibits functional specificity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Similar articles
-
Myoepithelial cell contraction and milk ejection are impaired in mammary glands of mice lacking smooth muscle alpha-actin.Biol Reprod. 2011 Jul;85(1):13-21. doi: 10.1095/biolreprod.110.090639. Epub 2011 Mar 2. Biol Reprod. 2011. PMID: 21368298 Free PMC article.
-
Asymmetric expression of connexins between luminal epithelial- and myoepithelial- cells is essential for contractile function of the mammary gland.Dev Biol. 2015 Mar 1;399(1):15-26. doi: 10.1016/j.ydbio.2014.11.026. Epub 2014 Dec 11. Dev Biol. 2015. PMID: 25500615 Free PMC article.
-
Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells.Mol Cell Biol. 2006 Aug;26(15):5797-808. doi: 10.1128/MCB.00211-06. Mol Cell Biol. 2006. PMID: 16847332 Free PMC article.
-
α-Smooth Muscle Actin and ACTA2 Gene Expressions in Vasculopathies.Braz J Cardiovasc Surg. 2015 Nov-Dec;30(6):644-9. doi: 10.5935/1678-9741.20150081. Braz J Cardiovasc Surg. 2015. PMID: 26934405 Free PMC article. Review.
-
The molecular culprits underlying precocious mammary gland involution.J Mammary Gland Biol Neoplasia. 2007 Mar;12(1):15-23. doi: 10.1007/s10911-007-9034-8. J Mammary Gland Biol Neoplasia. 2007. PMID: 17323120 Review.
Cited by
-
Smooth muscle α actin (Acta2) and myofibroblast function during hepatic wound healing.PLoS One. 2013 Oct 29;8(10):e77166. doi: 10.1371/journal.pone.0077166. eCollection 2013. PLoS One. 2013. PMID: 24204762 Free PMC article.
-
Whole animal knockout of smooth muscle alpha-actin does not alter excisional wound healing or the fibroblast-to-myofibroblast transition.Wound Repair Regen. 2013 Jan-Feb;21(1):166-76. doi: 10.1111/wrr.12001. Epub 2012 Dec 18. Wound Repair Regen. 2013. PMID: 23253249 Free PMC article.
-
Myoepithelial cells are a dynamic barrier to epithelial dissemination.J Cell Biol. 2018 Oct 1;217(10):3368-3381. doi: 10.1083/jcb.201802144. Epub 2018 Jul 30. J Cell Biol. 2018. PMID: 30061105 Free PMC article.
-
An Integrative Single-cell Transcriptomic Atlas of the Post-natal Mouse Mammary Gland Allows Discovery of New Developmental Trajectories in the Luminal Compartment.J Mammary Gland Biol Neoplasia. 2021 Mar;26(1):29-42. doi: 10.1007/s10911-021-09488-1. Epub 2021 Apr 28. J Mammary Gland Biol Neoplasia. 2021. PMID: 33913090
-
RUNX1, a transcription factor mutated in breast cancer, controls the fate of ER-positive mammary luminal cells.Elife. 2014 Nov 21;3:e03881. doi: 10.7554/eLife.03881. Elife. 2014. PMID: 25415051 Free PMC article.
References
-
- Abdelwahid E, Pelliniemi LJ, Szucsik JC, Lessard JL, Jokinen E. Cellular disorganization and extensive apoptosis in the developing heart of mice that lack cardiac muscle alpha-actin: apparent cause of perinatal death. Pediatr Res. 2004;55:197–204. - PubMed
-
- Barja F, Coughlin C, Belin D, Gabbiani G. Actin isoform synthesis and mRNA levels in quiescent and proliferating rat aortic smooth muscle cells in vivo and in vitro. Lab Invest. 1986;55:226–33. - PubMed
-
- Bruckmaier RM, Wellnitz O. Induction of milk ejection and milk removal in different production systems. J Anim Sci. 2008;86:15–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

