Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders
- PMID: 22123062
- PMCID: PMC3242840
- DOI: 10.4161/mabs.3.6.17815
Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders
Abstract
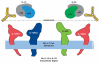
Monoclonal antibody (mAb) therapy was first established upon the approval of a mouse antibody for treatment of human acute organ rejection. However, the high incidence of immune response against the mouse mAb restricted therapeutic utility. Development of chimeric, "humanized" and human mAbs broadened therapeutic application to immune-mediated diseases requiring long-term treatment. Indeed, mAb therapeutics targeting soluble cytokines are highly effective in numerous immune-mediated disorders. A recent example is ustekinumab, a first-in-class therapeutic human immunoglobulin G1 kappa mAb that binds to the interleukins (IL)-12 and IL-23, cytokines that modulate lymphocyte function, including T-helper (Th) 1 and Th17 cell subsets. Ustekinumab was generated via recombinant human IL-12 immunization of human immunoglobulin (hu-Ig) transgenic mice. Ustekinumab binds to the p40 subunit common to IL-12 and IL-23 and prevents their interaction with the IL-12 receptor β1 subunit of the IL-12 and IL-23 receptor complexes. Ustekinumab is approved for treatment of moderate-to-severe plaque psoriasis and has demonstrated efficacy in Crohn disease and psoriatic arthritis. The clinical characterization of ustekinumab continues to clarify our understanding of human immune pathologies and may offer a novel therapeutic option for certain immune-mediated diseases.
Figures

References
-
- Winau F, Westphal O, Winau R. Paul Ehrlich—in search of a magic bullet. Microbes Infect. 2004;6:786–789. - PubMed
-
- Raju TN. The Nobel chronicles. 1972: Gerald M Edelman (b 1929) and Rodney R Porter (1917–85) Lancet. 1999;354:1040. - PubMed
-
- Orthoclone OKT®3 prescribing information. www.janssenbiotech.com/assets/OKT3_PI.pdf.
-
- Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
