Norcantharidin induces melanoma cell apoptosis through activation of TR3 dependent pathway
- PMID: 22123174
- PMCID: PMC3280911
- DOI: 10.4161/cbt.12.11.18380
Norcantharidin induces melanoma cell apoptosis through activation of TR3 dependent pathway
Abstract
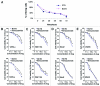
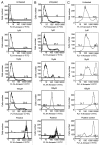
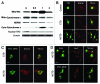
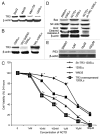
Norcantharidin (NCTD) has been reported to induce tumor cell apoptosis. However, the underlying mechanism behinds its antitumor effect remains elusive. We have previously shown that TR3 expression is significantly decreased in metastatic melanomas and involved in melanoma cell apoptosis. In this study, we showed that NCTD inhibited melanoma cell proliferation and induced apoptosis in a dose related manner. NCTD induced translocation of TR3 from nucleus to mitochondria where it co-localized with Bcl-2 in melanoma cells. NCTD also increased cytochome c release from mitochondria to the cytoplasm. These changes were accompanied by increased expression of Bax and cleaved caspase-3 along with decreased expression of Bcl2 and NF-κB2. The effects of NCTD were inhibited by knockdown of TR3 expression using TR3 specific shRNA in melanoma cells. Furthermore, NCTD significantly decreased tumor volume and improved survival of Tyr::CreER; BRAF(Ca/+); Pten(lox/lox) transgenic mice. Our data indicates that NCTD inhibits melanoma growth by inducing tumor cell apoptosis via activation of a TR3 dependent pathway. These results suggest that NCTD is a potential therapeutic agent for melanoma.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
