Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation
- PMID: 22123186
- PMCID: PMC3326563
- DOI: 10.1186/bcr3067
Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers--role of estrogen receptor and HER2 reactivation
Abstract
Introduction: The human epidermal growth factor receptor 2 (HER2)-targeted therapies trastuzumab (T) and lapatinib (L) show high efficacy in patients with HER2-positive breast cancer, but resistance is prevalent. Here we investigate resistance mechanisms to each drug alone, or to their combination using a large panel of HER2-positive cell lines made resistant to these drugs.
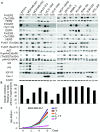
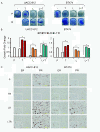
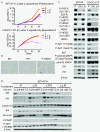
Methods: Response to L + T treatment was characterized in a panel of 13 HER2-positive cell lines to identify lines that were de novo resistant. Acquired resistant lines were then established by long-term exposure to increasing drug concentrations. Levels and activity of HER2 and estrogen receptor (ER) pathways were determined by qRT-PCR, immunohistochemistry, and immunoblotting assays. Cell growth, proliferation, and apoptosis in parental cells and resistant derivatives were assessed in response to inhibition of HER or ER pathways, either pharmacologically (L, T, L + T, or fulvestrant) or by using siRNAs. Efficacy of combined endocrine and anti-HER2 therapies was studied in vivo using UACC-812 xenografts.
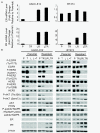
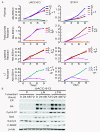
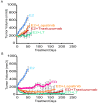
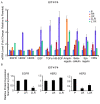
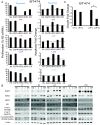
Results: ER or its downstream products increased in four out of the five ER+/HER2+ lines, and was evident in one of the two intrinsically resistant lines. In UACC-812 and BT474 parental and resistant derivatives, HER2 inhibition by T reactivated HER network activity to promote resistance. T-resistant lines remained sensitive to HER2 inhibition by either L or HER2 siRNA. With more complete HER2 blockade, resistance to L-containing regimens required the activation of a redundant survival pathway, ER, which was up-regulated and promoted survival via various Bcl2 family members. These L- and L + T-resistant lines were responsive to fulvestrant and to ER siRNA. However, after prolonged treatment with L, but not L + T, BT474 cells switched from depending on ER as a survival pathway, to relying again on the HER network (increased HER2, HER3, and receptor ligands) to overcome L's effects. The combination of endocrine and L + T HER2-targeted therapies achieved complete tumor regression and prevented development of resistance in UACC-812 xenografts.
Conclusions: Combined L + T treatment provides a more complete and stable inhibition of the HER network. With sustained HER2 inhibition, ER functions as a key escape/survival pathway in ER-positive/HER2-positive cells. Complete blockade of the HER network, together with ER inhibition, may provide optimal therapy in selected patients.
Figures
References
-
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

