Validation of the essential ClpP protease in Mycobacterium tuberculosis as a novel drug target
- PMID: 22123255
- PMCID: PMC3264079
- DOI: 10.1128/JB.06142-11
Validation of the essential ClpP protease in Mycobacterium tuberculosis as a novel drug target
Abstract
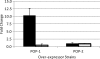
Mycobacterium tuberculosis is a pathogen of major global importance. Validated drug targets are required in order to develop novel therapeutics for drug-resistant strains and to shorten therapy. The Clp protease complexes provide a means for quality control of cellular proteins; the proteolytic activity of ClpP in concert with the ATPase activity of the ClpX/ClpC subunits results in degradation of misfolded or damaged proteins. Thus, the Clp system plays a major role in basic metabolism, as well as in stress responses and pathogenic mechanisms. M. tuberculosis has two ClpP proteolytic subunits. Here we demonstrate that ClpP1 is essential for viability in this organism in culture, since the gene could only be deleted from the chromosome when a second functional copy was provided. Overexpression of clpP1 had no effect on growth in aerobic culture or viability under anaerobic conditions or during nutrient starvation. In contrast, clpP2 overexpression was toxic, suggesting different roles for the two homologs. We synthesized known activators of ClpP protease activity; these acyldepsipeptides (ADEPs) were active against M. tuberculosis. ADEP activity was enhanced by the addition of efflux pump inhibitors, demonstrating that ADEPs gain access to the cell but that export occurs. Taken together, the genetic and chemical validation of ClpP as a drug target leads to new avenues for drug discovery.
Figures
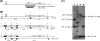
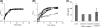
Similar articles
-
The Mycobacterium tuberculosis ClpP1P2 Protease Interacts Asymmetrically with Its ATPase Partners ClpX and ClpC1.PLoS One. 2015 May 1;10(5):e0125345. doi: 10.1371/journal.pone.0125345. eCollection 2015. PLoS One. 2015. PMID: 25933022 Free PMC article.
-
Antibiotic Acyldepsipeptides Stimulate the Streptomyces Clp-ATPase/ClpP Complex for Accelerated Proteolysis.mBio. 2022 Dec 20;13(6):e0141322. doi: 10.1128/mbio.01413-22. Epub 2022 Oct 26. mBio. 2022. PMID: 36286522 Free PMC article.
-
The ClpX and ClpP2 Orthologs of Chlamydia trachomatis Perform Discrete and Essential Functions in Organism Growth and Development.mBio. 2020 Sep 1;11(5):e02016-20. doi: 10.1128/mBio.02016-20. mBio. 2020. PMID: 32873765 Free PMC article.
-
The development of small-molecule modulators for ClpP protease activity.Mol Biosyst. 2016 Dec 20;13(1):23-31. doi: 10.1039/c6mb00644b. Mol Biosyst. 2016. PMID: 27831584 Review.
-
Harnessing caseinolytic proteases ClpP1P2 for next-generation antimycobacterial agents: Mechanisms, challenges and opportunities.Eur J Med Chem. 2025 Oct 15;296:117858. doi: 10.1016/j.ejmech.2025.117858. Epub 2025 Jun 19. Eur J Med Chem. 2025. PMID: 40582187 Review.
Cited by
-
Reprogramming of the Caseinolytic Protease by ADEP Antibiotics: Molecular Mechanism, Cellular Consequences, Therapeutic Potential.Front Mol Biosci. 2021 May 13;8:690902. doi: 10.3389/fmolb.2021.690902. eCollection 2021. Front Mol Biosci. 2021. PMID: 34109219 Free PMC article. Review.
-
Restriction of the conformational dynamics of the cyclic acyldepsipeptide antibiotics improves their antibacterial activity.J Am Chem Soc. 2014 Feb 5;136(5):1922-9. doi: 10.1021/ja410385c. Epub 2014 Jan 24. J Am Chem Soc. 2014. PMID: 24422534 Free PMC article.
-
Non-monotonic Response to Monotonic Stimulus: Regulation of Glyoxylate Shunt Gene-Expression Dynamics in Mycobacterium tuberculosis.PLoS Comput Biol. 2016 Feb 22;12(2):e1004741. doi: 10.1371/journal.pcbi.1004741. eCollection 2016 Feb. PLoS Comput Biol. 2016. PMID: 26900694 Free PMC article.
-
Protein-coding housekeeping gene Rv2461c can be used as an amplification target in loop-mediated isothermal amplification assay for the detection of Mycobacterium tuberculosis in sputum samples.Int J Clin Exp Pathol. 2014 Dec 1;7(12):8706-14. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25674236 Free PMC article.
-
Exploring anti-bacterial compounds against intracellular Legionella.PLoS One. 2013 Sep 13;8(9):e74813. doi: 10.1371/journal.pone.0074813. eCollection 2013. PLoS One. 2013. PMID: 24058631 Free PMC article.
References
-
- Balganesh TS, Furr NJ. 2007. Molecular approaches to target discovery: evaluating targets for anti-tuberculosis drug discovery programmes. Infect. Disord. Drug Targets 7:120–126 - PubMed
-
- Barik S, Sureka K, Mukherjee K, Basu J, Kundu M. 2010. RseA, the SigE specific anti-sigma factor of Mycobacterium tuberculosis, is inactivated by phosphorylation-dependent ClpC1P2 proteolysis. Mol. Microbiol. 75:592–606 - PubMed
-
- Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717–731 - PubMed
-
- Brötz-Oesterhelt H, et al. 2005. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 11:1082–1087 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

