Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS)
- PMID: 22123412
- PMCID: PMC3264832
- DOI: 10.1016/j.bbr.2011.11.018
Genetic reduction of muscarinic M4 receptor modulates analgesic response and acoustic startle response in a mouse model of fragile X syndrome (FXS)
Abstract
Introduction: The G-protein coupled muscarinic acetylcholine receptors, widely expressed in the CNS, have been implicated in fragile X syndrome (FXS). Recent studies have reported an overactive signaling through the muscarinic receptors in the Fmr1KO mouse model. Hence, it was hypothesized that reducing muscarinic signaling might modulate behavioral phenotypes in the Fmr1KO mice. Pharmacological studies from our lab have provided evidence for this hypothesis, with subtype-preferring muscarinic M1 and M4 receptor antagonists modulating select behaviors in the Fmr1KO mice. Since the pharmacological antagonists were not highly specific, we investigated the specific role of M4 receptors in the Fmr1KO mouse model, using a genetic approach.
Methods: We created a double mutant heterozygous for the M4 receptor gene and hemizygous for the Fmr1 gene and examined the mutants on various behaviors. Each animal was tested on a behavior battery comprising of open-field activity (activity), light-dark (anxiety), marble burying (perseverative behavior), prepulse inhibition (sensorimotor gating), rotarod (motor coordination), passive avoidance (learning and memory) and hotplate (analgesia). Animals were also tested on the audiogenic seizure protocol and testis weights were measured.
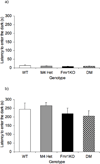
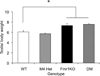
Results: Reduction of M4 receptor expression in the heterozygotes completely rescued the analgesic response and partly rescued the acoustic startle response phenotype in the Fmr1KO mice. However, no modulation was observed in a number of behaviors including learning and memory, activity, perseverative behavior and audiogenic seizures.
Conclusion: Reducing M4 receptor signaling altered only select behavioral phenotypes in the Fmr1KO mouse model, suggesting that other targets are involved in the modulation of fragile X behaviors.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




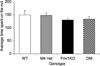

Similar articles
-
The modulation of fragile X behaviors by the muscarinic M4 antagonist, tropicamide.Behav Neurosci. 2011 Oct;125(5):783-90. doi: 10.1037/a0025202. Behav Neurosci. 2011. PMID: 21942438 Free PMC article.
-
Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile X syndrome.Psychopharmacology (Berl). 2011 Sep;217(1):143-51. doi: 10.1007/s00213-011-2276-6. Epub 2011 Apr 13. Psychopharmacology (Berl). 2011. PMID: 21487657
-
Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome.Behav Brain Res. 2011 Oct 1;223(2):310-21. doi: 10.1016/j.bbr.2011.04.049. Epub 2011 May 6. Behav Brain Res. 2011. PMID: 21571007 Free PMC article.
-
Social behavior phenotypes in fragile X syndrome, autism, and the Fmr1 knockout mouse: theoretical comment on McNaughton et al. (2008).Behav Neurosci. 2008 Apr;122(2):483-9. doi: 10.1037/0735-7044.122.2.483. Behav Neurosci. 2008. PMID: 18410188 Review.
-
Learning and behavioral deficits associated with the absence of the fragile X mental retardation protein: what a fly and mouse model can teach us.Learn Mem. 2014 Sep 16;21(10):543-55. doi: 10.1101/lm.035956.114. Print 2014 Oct. Learn Mem. 2014. PMID: 25227249 Free PMC article. Review.
Cited by
-
Progress toward treatments for synaptic defects in autism.Nat Med. 2013 Jun;19(6):685-94. doi: 10.1038/nm.3193. Epub 2013 Jun 6. Nat Med. 2013. PMID: 23744158 Review.
-
Emerging pharmacologic treatment options for fragile X syndrome.Appl Clin Genet. 2015 Apr 7;8:75-93. doi: 10.2147/TACG.S35673. eCollection 2015. Appl Clin Genet. 2015. PMID: 25897255 Free PMC article. Review.
-
Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background.Behav Brain Res. 2014 Sep 1;271:72-8. doi: 10.1016/j.bbr.2014.05.046. Epub 2014 Jun 2. Behav Brain Res. 2014. PMID: 24886775 Free PMC article.
-
Total RNA Sequencing of Rett Syndrome Autopsy Samples Identifies the M4 Muscarinic Receptor as a Novel Therapeutic Target.J Pharmacol Exp Ther. 2018 May;365(2):291-300. doi: 10.1124/jpet.117.246991. Epub 2018 Mar 9. J Pharmacol Exp Ther. 2018. PMID: 29523700 Free PMC article.
-
Cell-Type-Specific Translation Profiling Reveals a Novel Strategy for Treating Fragile X Syndrome.Neuron. 2017 Aug 2;95(3):550-563.e5. doi: 10.1016/j.neuron.2017.07.013. Neuron. 2017. PMID: 28772121 Free PMC article.
References
-
- Hagerman RJ, Hagerman PJ. Fragile × syndrome: Diagnosis, treatment, and research. 3rd ed. Baltimore: The Johns Hopkins University Press; 2002.
-
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, et al. Fmr1 knockout mice: A model to study fragile × mental retardation. Cell. 1994;78(1):23–33. - PubMed
-
- Chen L, Toth M. Fragile × mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103(4):1043–1050. - PubMed
-
- Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100(2):423–429. - PubMed
-
- D'Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76(2):367–376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

