Subversion of membrane transport pathways by vacuolar pathogens
- PMID: 22123831
- PMCID: PMC3241728
- DOI: 10.1083/jcb.201105019
Subversion of membrane transport pathways by vacuolar pathogens
Abstract
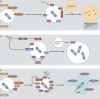
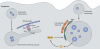
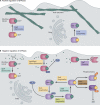
Mammalian phagocytes control bacterial infections effectively through phagocytosis, the process by which particles engulfed at the cell surface are transported to lysosomes for destruction. However, intracellular pathogens have evolved mechanisms to avoid this fate. Many bacterial pathogens use specialized secretion systems to deliver proteins into host cells that subvert signaling pathways controlling membrane transport. These bacterial effectors modulate the function of proteins that regulate membrane transport and alter the phospholipid content of membranes. Elucidating the biochemical function of these effectors has provided a greater understanding of how bacteria control membrane transport to create a replicative niche within the host and provided insight into the regulation of membrane transport in eukaryotic cells.
Figures
References
-
- Bakowski M.A., Braun V., Lam G.Y., Yeung T., Heo W.D., Meyer T., Finlay B.B., Grinstein S., Brumell J.H. 2010. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe. 7:453–462 10.1016/j.chom.2010.05.011 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

