The cell biology of receptor-mediated virus entry
- PMID: 22123832
- PMCID: PMC3246895
- DOI: 10.1083/jcb.201108131
The cell biology of receptor-mediated virus entry
Abstract
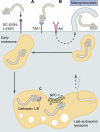
The cell imposes multiple barriers to virus entry. However, viruses exploit fundamental cellular processes to gain entry to cells and deliver their genetic cargo. Virus entry pathways are largely defined by the interactions between virus particles and their receptors at the cell surface. These interactions determine the mechanisms of virus attachment, uptake, intracellular trafficking, and, ultimately, penetration to the cytosol. Elucidating the complex interplay between viruses and their receptors is necessary for a full understanding of how these remarkable agents invade their cellular hosts.
Figures


References
-
- Barth H., Schafer C., Adah M.I., Zhang F., Linhardt R.J., Toyoda H., Kinoshita-Toyoda A., Toida T., Van Kuppevelt T.H., Depla E., et al. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003–41012 10.1074/jbc.M302267200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

