Cell cycle regulation in hematopoietic stem cells
- PMID: 22123859
- PMCID: PMC3257565
- DOI: 10.1083/jcb.201102131
Cell cycle regulation in hematopoietic stem cells
Abstract
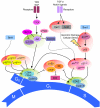
Hematopoietic stem cells (HSCs) give rise to all lineages of blood cells. Because HSCs must persist for a lifetime, the balance between their proliferation and quiescence is carefully regulated to ensure blood homeostasis while limiting cellular damage. Cell cycle regulation therefore plays a critical role in controlling HSC function during both fetal life and in the adult. The cell cycle activity of HSCs is carefully modulated by a complex interplay between cell-intrinsic mechanisms and cell-extrinsic factors produced by the microenvironment. This fine-tuned regulatory network may become altered with age, leading to aberrant HSC cell cycle regulation, degraded HSC function, and hematological malignancy.
Figures