A randomised controlled pilot study of standardised counselling and cost-free pharmacotherapy for smoking cessation among stroke and TIA patients
- PMID: 22123923
- PMCID: PMC3225588
- DOI: 10.1136/bmjopen-2011-000366
A randomised controlled pilot study of standardised counselling and cost-free pharmacotherapy for smoking cessation among stroke and TIA patients
Abstract
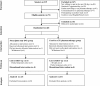
Background Tobacco use is a major risk factor for recurrent stroke. The provision of cost-free quit smoking medications has been shown to be efficacious in increasing smoking abstinence in the general population. Objective The objective of this pilot study was to assess the feasibility and obtain preliminary data on the effectiveness of providing cost-free quit smoking pharmacotherapy and counselling to smokers identified in a stroke prevention clinic. Trial design Cluster randomised controlled trial. Methods All patients seen at the Ottawa Hospital Stroke Prevention Clinic who smoked more five or more cigarettes per day, were ready to quit smoking in the next 30 days, and were willing to use pharmacotherapy were invited to participate in the study. All participants were advised to quit smoking and treated using a standardised protocol including counselling and pharmacotherapy. Participants were randomly assigned to either a prescription only usual care group or an experimental group who received a 4-week supply of cost-free quit smoking medications and a prescription for medication renewal. All patients received follow-up counselling. The primary outcome was biochemically validated quit rates at 26 weeks. The research coordinator conducting outcome assessment was blind to group allocation. Results Of 219 smokers screened, 73 were eligible, 28 consented and were randomised, and 25 completed the 26-week follow-up assessment. All 28 patients randomised were included in the analysis. The biochemically validated 7-day point prevalence abstinence rate in the experimental group compared to the usual care group was 26.6% vs 15.4% (adjusted OR 2.00, 95% CI 0.33 to 13.26; p=0.20). Conclusions It would be feasible to definitively evaluate this intervention in a large multi-site trial. Trial registration number http://ClinicalTrials.gov # UOHI2010-1.
Conflict of interest statement
Figures
References
-
- Wolf PA, D'Agostino RB, Kannel WB, et al. Cigarette smoking as a risk factor for stroke. The Framingham Study. JAMA 1988;259:1025–9 - PubMed
-
- Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation in relation to total mortality rates in women. A prospective cohort study. Ann Intern Med 1993;119:992–1000 - PubMed
-
- Ovbiagele B, Saver JL, Fredieu A, et al. In-hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow-up. Stroke 2004;35:2879–83 - PubMed
-
- Wannamethee SG, Shaper AG, Whincup PH, et al. Smoking cessation and the risk of stroke in middle-aged men. JAMA 1995;274:155–60 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical