Robo4 regulates the radial migration of newborn neurons in developing neocortex
- PMID: 22123939
- PMCID: PMC4705339
- DOI: 10.1093/cercor/bhr330
Robo4 regulates the radial migration of newborn neurons in developing neocortex
Abstract
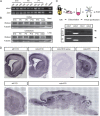
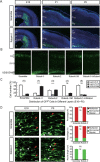
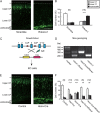
During the morphogenesis of neocortex, newborn neurons undergo radial migration from the ventricular zone toward the surface of the cortical plate to form an "inside-out" lamina structure. The spatiotemporal signals that control this stereotyped radial migration remain elusive. Here, we report that a recently identified Robo family member Robo4 (Magic Roundabout), which was considered to be solely expressed in endothelial cells, is expressed in developing brain and regulates the radial migration of newborn neurons in neocortex. Downregulation of Robo4 expression in cortical newborn neurons by using in utero electroporation, with either specific siRNAs in wild-type rodents or with Cre recombinase in floxed-robo4 mutant mice, led to severe defects in the radial migration of newborn neurons with misorientation of these neurons. Moreover, newborn neurons transfected with Robo4 siRNAs exhibited significantly lower motility in a transwell migration assay (Boyden chamber) in the absence of Slit and significantly higher sensitivity to the repulsive effect of Slit in both transwell migration assay and growth cone collapse assay. Overall, our results showed an important role of Robo4 in the regulation of cortical radial migration through Slit-dependent and -independent mechanisms.
Figures



Similar articles
-
Robo1 modulates proliferation and neurogenesis in the developing neocortex.J Neurosci. 2014 Apr 16;34(16):5717-31. doi: 10.1523/JNEUROSCI.4256-13.2014. J Neurosci. 2014. PMID: 24741061 Free PMC article.
-
Defective neuronal migration and inhibition of bipolar to multipolar transition of migrating neural cells by Mesoderm-Specific Transcript, Mest, in the developing mouse neocortex.Neuroscience. 2017 Jul 4;355:126-140. doi: 10.1016/j.neuroscience.2017.05.003. Epub 2017 May 10. Neuroscience. 2017. PMID: 28501506
-
Robo4 is a vascular-specific receptor that inhibits endothelial migration.Dev Biol. 2003 Sep 1;261(1):251-67. doi: 10.1016/s0012-1606(03)00258-6. Dev Biol. 2003. PMID: 12941633
-
Regulatory mechanisms of Robo4 and their effects on angiogenesis.Biosci Rep. 2019 Jul 10;39(7):BSR20190513. doi: 10.1042/BSR20190513. Print 2019 Jul 31. Biosci Rep. 2019. PMID: 31160487 Free PMC article. Review.
-
Molecular regulation of neuronal migration during neocortical development.Mol Cell Neurosci. 2009 Sep;42(1):11-22. doi: 10.1016/j.mcn.2009.06.003. Epub 2009 Jun 10. Mol Cell Neurosci. 2009. PMID: 19523518 Review.
Cited by
-
c-Jun NH2-terminal kinase (JNK)-interacting protein-3 (JIP3) regulates neuronal axon elongation in a kinesin- and JNK-dependent manner.J Biol Chem. 2013 May 17;288(20):14531-14543. doi: 10.1074/jbc.M113.464453. Epub 2013 Apr 10. J Biol Chem. 2013. PMID: 23576431 Free PMC article.
-
Spatiotemporal Regulation of Rho GTPases in Neuronal Migration.Cells. 2019 Jun 10;8(6):568. doi: 10.3390/cells8060568. Cells. 2019. PMID: 31185627 Free PMC article. Review.
-
The Role of the Slit/Robo Signaling Pathway.J Cancer. 2019 Jun 2;10(12):2694-2705. doi: 10.7150/jca.31877. eCollection 2019. J Cancer. 2019. PMID: 31258778 Free PMC article. Review.
-
Making Connections: Guidance Cues and Receptors at Nonneural Cell-Cell Junctions.Cold Spring Harb Perspect Biol. 2018 Nov 1;10(11):a029165. doi: 10.1101/cshperspect.a029165. Cold Spring Harb Perspect Biol. 2018. PMID: 28847900 Free PMC article. Review.
-
The Slit-Robo signalling pathway in nervous system development: a comparative perspective from vertebrates and invertebrates.Open Biol. 2025 Jul;15(7):250026. doi: 10.1098/rsob.250026. Epub 2025 Jul 9. Open Biol. 2025. PMID: 40628293 Free PMC article. Review.
References
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. - PubMed
-
- Angevine JB, Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. - PubMed
-
- Anitha A, Nakamura K, Yamada K, Suda S, Thanseem I, Tsujii M, Iwayama Y, Hattori E, Toyota T, Miyachi T, et al. Genetic analyses of roundabout (ROBO) axon guidance receptors in autism. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1019–1027. - PubMed
-
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

