Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells
- PMID: 22123951
- PMCID: PMC3251070
- DOI: 10.1073/pnas.1115050108
Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells
Abstract
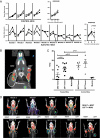
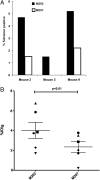
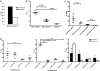
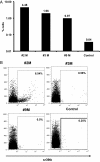
The goal of cancer immunotherapy is the generation of an effective, stable, and self-renewing antitumor T-cell population. One such approach involves the use of high-affinity cancer-specific T-cell receptors in gene-therapy protocols. Here, we present the generation of functional tumor-specific human T cells in vivo from genetically modified human hematopoietic stem cells (hHSC) using a human/mouse chimera model. Transduced hHSC expressing an HLA-A*0201-restricted melanoma-specific T-cell receptor were introduced into humanized mice, resulting in the generation of a sizeable melanoma-specific naïve CD8(+) T-cell population. Following tumor challenge, these transgenic CD8(+) T cells, in the absence of additional manipulation, limited and cleared human melanoma tumors in vivo. Furthermore, the genetically enhanced T cells underwent proper thymic selection, because we did not observe any responses against non-HLA-matched tumors, and no killing of any kind occurred in the absence of a human thymus. Finally, the transduced hHSC established long-term bone marrow engraftment. These studies present a potential therapeutic approach and an important tool to understand better and to optimize the human immune response to melanoma and, potentially, to other types of cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–342. - PubMed
-
- Ochsenbein AF. Principles of tumor immunosurveillance and implications for immunotherapy. Cancer Gene Ther. 2002;9:1043–1055. - PubMed
-
- Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: Mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. - PubMed
-
- Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

