Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants
- PMID: 22123958
- PMCID: PMC3250165
- DOI: 10.1073/pnas.1111902108
Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants
Erratum in
- Proc Natl Acad Sci U S A. 2012 Aug 28;109(35):14277
Abstract
Long distance cell-to-cell communication is critical for the development of multicellular organisms. In this respect, plants are especially demanding as they constantly integrate environmental inputs to adjust growth processes to different conditions. One example is thickening of shoots and roots, also designated as secondary growth. Secondary growth is mediated by the vascular cambium, a stem cell-like tissue whose cell-proliferating activity is regulated over a long distance by the plant hormone auxin. How auxin signaling is integrated at the level of cambium cells and how cambium activity is coordinated with other growth processes are largely unknown. Here, we provide physiological, genetic, and pharmacological evidence that strigolactones (SLs), a group of plant hormones recently described to be involved in the repression of shoot branching, positively regulate cambial activity and that this function is conserved among species. We show that SL signaling in the vascular cambium itself is sufficient for cambium stimulation and that it interacts strongly with the auxin signaling pathway. Our results provide a model of how auxin-based long-distance signaling is translated into cambium activity and suggest that SLs act as general modulators of plant growth forms linking the control of shoot branching with the thickening of stems and roots.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
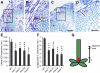
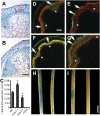
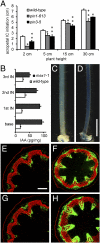
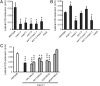
References
-
- Snow R. Activation of cambial growth by pure hormones. New Phytol. 1935;34:347–360.
-
- Turnbull CG, Booker JP, Leyser HM. Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J. 2002;32:255–262. - PubMed
-
- Umehara M, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. - PubMed
-
- Gomez-Roldan V, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

