High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp
- PMID: 22123974
- PMCID: PMC3248512
- DOI: 10.1073/pnas.1105861108
High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp
Abstract
Algae have reemerged as potential next-generation feedstocks for biofuels, but strain improvement and progress in algal biology research have been limited by the lack of advanced molecular tools for most eukaryotic microalgae. Here we describe the development of an efficient transformation method for Nannochloropsis sp., a fast-growing, unicellular alga capable of accumulating large amounts of oil. Moreover, we provide additional evidence that Nannochloropsis is haploid, and we demonstrate that insertion of transformation constructs into the nuclear genome can occur by high-efficiency homologous recombination. As examples, we generated knockouts of the genes encoding nitrate reductase and nitrite reductase, resulting in strains that were unable to grow on nitrate and nitrate/nitrite, respectively. The application of homologous recombination in this industrially relevant alga has the potential to rapidly advance algal functional genomics and biotechnology.
Conflict of interest statement
Conflict of interest statement: O.K., C.S.E.B., and B.V. are employees of Aurora Algae, Inc. K.K.N. is a member of the Scientific Advisory Board and has served as a consultant for Aurora Algae, Inc. Patent applications have been filed for some of the technology disclosed in this publication.
Figures
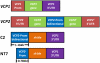


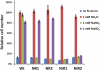
Comment in
-
Homologous recombination in Nannochloropsis: a powerful tool in an industrially relevant alga.Proc Natl Acad Sci U S A. 2011 Dec 27;108(52):20859-60. doi: 10.1073/pnas.1118670109. Epub 2011 Dec 19. Proc Natl Acad Sci U S A. 2011. PMID: 22184230 Free PMC article. No abstract available.
References
-
- Wijffels RH, Barbosa MJ. An outlook on microalgal biofuels. Science. 2010;329:796–799. - PubMed
-
- Stephens E, et al. An economic and technical evaluation of microalgal biofuels. Nat Biotechnol. 2010;28(2):126–128. - PubMed
-
- Stephens E, et al. Future prospects of microalgal biofuel production systems. Trends Plant Sci. 2010;15:554–564. - PubMed
-
- Scott SA, et al. Biodiesel from algae: Challenges and prospects. Curr Opin Biotechnol. 2010;21:277–286. - PubMed
-
- Hu Q, et al. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008;54:621–639. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

