Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group
- PMID: 22124095
- PMCID: PMC3383117
- DOI: 10.1200/JCO.2011.34.8987
Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children's Oncology Group
Abstract
Purpose: Carbonyl reductases (CBRs) catalyze reduction of anthracyclines to cardiotoxic alcohol metabolites. Polymorphisms in CBR1 and CBR3 influence synthesis of these metabolites. We examined whether single nucleotide polymorphisms in CBR1 (CBR1 1096G>A) and/or CBR3 (CBR3 V244M) modified the dose-dependent risk of anthracycline-related cardiomyopathy in childhood cancer survivors.
Patients and methods: One hundred seventy survivors with cardiomyopathy (patient cases) were compared with 317 survivors with no cardiomyopathy (controls; matched on cancer diagnosis, year of diagnosis, length of follow-up, and race/ethnicity) using conditional logistic regression techniques.
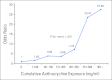
Results: A dose-dependent association was observed between cumulative anthracycline exposure and cardiomyopathy risk (0 mg/m(2): reference; 1 to 100 mg/m(2): odds ratio [OR], 1.65; 101 to 150 mg/m(2): OR, 3.85; 151 to 200 mg/m(2): OR, 3.69; 201 to 250 mg/m(2): OR, 7.23; 251 to 300 mg/m(2): OR, 23.47; > 300 mg/m(2): OR, 27.59; P(trend) < .001). Among individuals carrying the variant A allele (CBR1:GA/AA and/or CBR3:GA/AA), exposure to low- to moderate-dose anthracyclines (1 to 250 mg/m(2)) did not increase the risk of cardiomyopathy. Among individuals with CBR3 V244M homozygous G genotypes (CBR3:GG), exposure to low- to moderate-dose anthracyclines increased cardiomyopathy risk when compared with individuals with CBR3:GA/AA genotypes unexposed to anthracyclines (OR, 5.48; P = .003), as well as exposed to low- to moderate-dose anthracyclines (OR, 3.30; P = .006). High-dose anthracyclines (> 250 mg/m(2)) were associated with increased cardiomyopathy risk, irrespective of CBR genotype status.
Conclusion: This study demonstrates increased anthracycline-related cardiomyopathy risk at doses as low as 101 to 150 mg/m(2). Homozygosis for G allele in CBR3 contributes to increased cardiomyopathy risk associated with low- to moderate-dose anthracyclines, such that there seems to be no safe dose for patients homozygous for the CBR3 V244M G allele. These results suggest a need for targeted intervention for those at increased risk of cardiomyopathy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Getting to the heart of the matter.J Clin Oncol. 2012 May 1;30(13):1399-400. doi: 10.1200/JCO.2011.40.1422. Epub 2012 Apr 2. J Clin Oncol. 2012. PMID: 22473154 No abstract available.
-
Single nucleotide polymorphisms and anthracycline cardiotoxicity in children: potential implications for adult oncology.J Clin Oncol. 2012 Oct 1;30(28):3563; author reply 3563-4. doi: 10.1200/JCO.2012.44.6484. Epub 2012 Aug 27. J Clin Oncol. 2012. PMID: 22927524 No abstract available.
References
-
- Kremer LCM, Caron HN. Anthracycline cardiotoxicity in children. N Engl J Med. 2004;351:120–121. - PubMed
-
- Wouters KA, Kremer LCM, Miller TL, et al. Protecting against anthracycline-induced myocardial damage: A review of the most promising strategies. Br J Haematol. 2005;131:561–578. - PubMed
-
- Bryant J, Picot J, Levitt G, et al. Cardioprotection against the toxic effects of anthracyclines given to children with cancer: A systematic review. Health Technol Assess. 2007;11:1–84. - PubMed
-
- Kremer LCM, van der Pal HJH, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann Oncol. 2002;13:819–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

