BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma
- PMID: 22124101
- PMCID: PMC4669379
- DOI: 10.1200/JCO.2011.34.6270
BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma
Abstract
Purpose: Metastatic melanoma, a highly vascularized tumor with strong expression of vascular endothelial growth factor, has an overall poor prognosis. We conducted a placebo-controlled, double-blind phase II study of carboplatin plus paclitaxel with or without bevacizumab in patients with previously untreated metastatic melanoma.
Patients and methods: Patients were randomly assigned in a two-to-one ratio to carboplatin (area under the curve, 5) plus paclitaxel (175 mg/m(2)) and bevacizumab (15 mg/kg; CPB) or placebo (CP) administered intravenously once every 3 weeks. Progression-free survival (PFS) was the primary end point. Secondary end points included overall survival (OS) and safety.
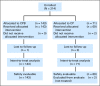
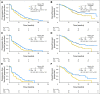
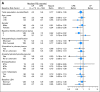
Results: Two hundred fourteen patients (73% with M1c disease) were randomly assigned. With a median follow-up of 13 months, median PFS was 4.2 months for the CP arm (n = 71) and 5.6 months for the CPB arm (n = 143; hazard ratio [HR], 0.78; P = .1414). Overall response rates were 16.4% and 25.5%, respectively (P = .1577). With 13-month follow-up, median OS was 8.6 months in the CP arm versus 12.3 months in the CPB arm (HR, 0.67; P = .0366), whereas in an evaluation 4 months later, it was 9.2 versus 12.3 months, respectively (HR, 0.79; P = .1916). In patients with elevated serum lactate dehydrogenase (n = 84), median PFS and OS were longer in the CPB arm (PFS: 4.4 v 2.7 months; HR, 0.62; OS: 8.5 v 7.5 months; HR, 0.52). No new safety signals were observed.
Conclusion: The study did not meet the primary objective of statistically significant improvement in PFS with the addition of bevacizumab to carboplatin plus paclitaxel. A larger phase III study will be necessary to determine whether there is benefit to the addition of bevacizumab to carboplatin plus paclitaxel in this disease setting.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
BEAM trial: lighting the way ahead?J Clin Oncol. 2012 Jan 1;30(1):6-7. doi: 10.1200/JCO.2011.37.5832. Epub 2011 Nov 28. J Clin Oncol. 2012. PMID: 22124100 No abstract available.
-
Bevacizumab advanced melanoma (BEAM) was a positive trial.J Clin Oncol. 2012 Jun 1;30(16):2023; author reply 2023-4. doi: 10.1200/JCO.2012.41.7477. Epub 2012 Apr 23. J Clin Oncol. 2012. PMID: 22529260 No abstract available.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. - PubMed
-
- Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. - PubMed
-
- Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: A phase III study. J Clin Oncol. 2004;22:1118–1125. - PubMed
-
- Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

