Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors
- PMID: 22124109
- PMCID: PMC3295557
- DOI: 10.1200/JCO.2010.33.8889
Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors
Abstract
Purpose: We evaluated whether patients with human epidermal growth factor receptor 2 (HER2) -positive primary breast tumors had metastatic tumors that were HER2 positive (concordant) or HER2 negative (discordant). We then evaluated whether treatment with trastuzumab or chemotherapy before biopsy of the metastasis had any effect on the rate of HER2 discordance. We also compared the overall survival durations of patients with HER2-concordant and -discordant tumors.
Patients and methods: We retrospectively identified all patients who initially had been diagnosed with HER2-positive (immunohistochemistry 3+ and/or fluorescent in situ hybridization positive) primary breast cancer between 1997 and 2008 at MD Anderson Cancer Center who also had metastatic tumor biopsy results available for review.
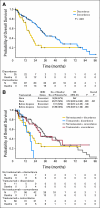
Results: We included 182 patients who met our criteria. Forty-three (24%) of the 182 patients with HER2-positive primary tumors had HER2-negative metastatic tumors. The HER2 discordance rates differed significantly on the basis of whether patients received chemotherapy (P = .022) but not on the basis of whether patients received trastuzumab (P = .296). Patients with discordant HER2 status had shorter overall survival than did patients with concordant HER2 status (hazard ratio [HR], 0.43; P = .003). A survival difference remained among the 67 patients who received trastuzumab (HR, 0.56; P = .083) and 101 patients who did not (HR, 0.53; P = .033) before their metastasis biopsies.
Conclusion: We confirmed that loss of HER2-positive status in metastatic tumors can occur in patients with primary HER2-positive breast cancer. Our data strongly support the need for biopsies of metastatic lesions to accurately determine patient prognosis and appropriate use of targeted therapy.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer.J Clin Oncol. 2012 Feb 20;30(6):587-92. doi: 10.1200/JCO.2010.33.5232. Epub 2011 Nov 28. J Clin Oncol. 2012. PMID: 22124102 Free PMC article.
References
-
- Masood S, Bui MM. Assessment of Her-2/neu overexpression in primary breast cancers and their metastatic lesions: An immunohistochemical study. Ann Clin Lab Sci. 2000;30:259–265. - PubMed
-
- Shimizu C, Fukutomi T, Tsuda H, et al. c-erbB-2 protein overexpression and p53 immunoreaction in primary and recurrent breast cancer tissues. J Surg Oncol. 2000;73:17–20. - PubMed
-
- Simon R, Nocito A, Hübscher T, et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J Natl Cancer Inst. 2001;93:1141–1146. - PubMed
-
- Tanner M, Järvinen P, Isola J. Amplification of HER-2/neu and topoisomerase IIalpha in primary and metastatic breast cancer. Cancer Res. 2001;61:5345–5348. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

