The core domain as the force sensor of the yeast mechanosensitive TRP channel
- PMID: 22124118
- PMCID: PMC3226973
- DOI: 10.1085/jgp.201110693
The core domain as the force sensor of the yeast mechanosensitive TRP channel
Abstract
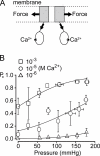
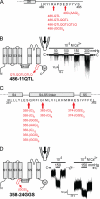
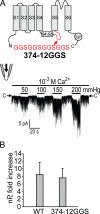
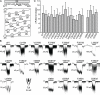
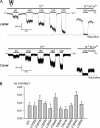
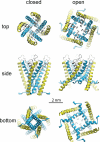
Stretch-activated conductances are commonly encountered in careful electric recordings. Those of known proteins (TRP, MscL, MscS, K(2p), Kv, etc.) all share a core, which houses the ion pathway and the gate, but no recognizable force-sensing domain. Like animal TRPs, the yeast TRPY1 is polymodal, activated by stretch force, Ca(2+), etc. To test whether its S5-S6 core senses the stretch force, we tried to uncouple it from the peripheral domains by strategic peptide insertions to block the covalent core-periphery interactions. Insertion of long unstructured peptides should distort, if not disrupt, protein structures that transmit force. Such insertions between S6 and the C-terminal tail largely removed Ca(2+) activation, showing their effectiveness. However, such insertions as well as those between S5 and the N-terminal region, which includes S1-S4, did not significantly alter mechanosensitivity. Even insertions at both locations flanking the S5-S6 core did not much alter mechanosensitivity. Tryptophan scanning mutations in S5 were also constructed to perturb possible noncovalent core-periphery contacts. The testable tryptophan mutations also have little or no effects on mechanosensitivity. Boltzmann fits of the wild-type force-response curves agree with a structural homology model for a stretch-induced core expansion of ~2 nm(2) upon opening. We hypothesize that membrane tension pulls on S5-S6, expanding the core and opening the TRPY1 gate. The core being the major force sensor offers the simplest, though not the only, explanation of why so many channels of disparate designs are mechanically sensitive. Compared with the bacterial MscL, TRPY1 is much less sensitive to force, befitting a polymodal channel that relies on multiple stimuli.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

