The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex
- PMID: 22124142
- PMCID: PMC3381319
- DOI: 10.1113/jphysiol.2011.219592
The early release of planned movement by acoustic startle can be delayed by transcranial magnetic stimulation over the motor cortex
Abstract
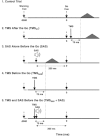
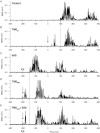
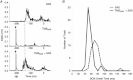
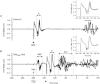
Previous studies have shown that preplanned movements can be rapidly released when a startling acoustic stimulus (SAS) is presented immediately prior to, or coincident with, the imperative signal to initiate movement. Based on the short latency of the onset of muscle activity (typically in less than 90 ms) and the frequent co-expression of startle responses in the neck and eye muscles, it has been proposed that the release of planned movements by a SAS is mediated by subcortical, possibly brainstem, pathways. However, a role for cortical structures in mediating these responses cannot be ruled out based on timing arguments alone. We examined the role of the cortex in the mediation of these responses by testing if a suprathreshold transcranial magnetic stimulation applied over the primary motor cortex, which suppresses voluntary drive and is known to delay movement initiation, could delay the release of movement by a SAS. Eight subjects performed an instructed-delay task requiring them to make a ballistic wrist movement to a target in response to an acoustic tone (control task condition). In a subset of trials subjects received one of the following: (1) suprathreshold TMS over the contralateral primary motor cortex 70 ms prior to their mean response time on control trials (TMS(CT)), (2) SAS 200 ms prior to the go cue (SAS), (3) suprathreshold TMS 70 ms prior to the mean SAS-evoked response time (TMS(SAS)), or (4) TMS(SAS) and SAS presented concurrently (TMS+SAS). Movement kinematics and EMG from the wrist extensors and flexors and sternocleidomastoid muscles were recorded. The application of TMS(CT) prior to control voluntary movements produced a significant delay in movement onset times (P < 0.001) (average delay = 37.7 ± 12.8 ms). The presentation of a SAS alone at -200 ms resulted in the release of the planned movement an average of 71.7 ± 2.7 ms after the startling stimulus. The early release of movement by a SAS was significantly delayed (P < 0.001, average delay = 35.0 ± 12.9 ms) when TMS(SAS) and SAS were presented concurrently. This delay could not be explained by a prolonged suppression of motor unit activity at the spinal level. These findings provide evidence that the release of targeted ballistic wrist movements by SAS is mediated, in part, by a fast conducting transcortical pathway via the primary motor cortex.
Figures


References
-
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res. 2004;159:284–300. - PubMed
-
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res. 2004a;159:301–309. - PubMed
-
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Mot Behav. 2004b;36:253–264. - PubMed
-
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res. 2007;176:199–205. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

