Measurement and simulation of myoplasmic calcium transients in mouse slow-twitch muscle fibres
- PMID: 22124146
- PMCID: PMC3379702
- DOI: 10.1113/jphysiol.2011.220780
Measurement and simulation of myoplasmic calcium transients in mouse slow-twitch muscle fibres
Abstract
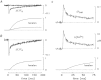
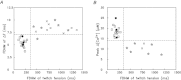
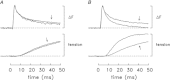
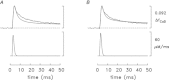
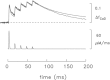
Bundles of intact fibres from soleus muscles of adult mice were isolated by dissection and one fibre within a bundle was micro-injected with either furaptra or mag-fluo-4, two low-affinity rapidly responding Ca(2+) indicators. Fibres were activated by action potentials to elicit changes in indicator fluorescence (ΔF), a monitor of the myoplasmic free Ca(2+) transient ([Ca(2+)]), and changes in fibre tension. All injected fibres appeared to be slow-twitch (type I) fibres as inferred from the time course of their tension responses. The full-duration at half-maximum (FDHM) of ΔF was found to be essentially identical with the two indicators; the mean value was 8.4 ± 0.3 ms (±SEM) at 16°C and 5.1 ± 0.3 ms at 22°C. The value at 22°C is about one-third that reported previously in enzyme-dissociated slow-twitch fibres that had been AM-loaded with mag-fluo-4: 12.4 ± 0.8 ms and 17.2 ± 1.7 ms. We attribute the larger FDHM in enzyme-dissociated fibres either to an alteration of fibre properties due to the enzyme treatment or to some error in the measurement of ΔF associated with AM loading. ΔF in intact fibres was simulated with a multi-compartment reaction-diffusion model that permitted estimation of the amount and time course of Ca(2+) release from the sarcoplasmic reticulum (SR), the binding and diffusion of Ca(2+) in the myoplasm, the re-uptake of Ca(2+) by the SR Ca(2+) pump, and Δ[Ca(2+)] itself. In response to one action potential at 16°C, the following estimates were obtained: 107 μm for the amount of Ca(2+) release; 1.7 ms for the FDHM of the release flux; 7.6 μm and 4.9 ms for the peak and FDHM of spatially averaged Δ[Ca(2+)]. With five action potentials at 67 Hz, the estimated amount of Ca(2+) release is 186 μm. Two important unknown model parameters are the on- and off-rate constants of the reaction between Ca(2+) and the regulatory sites on troponin; values of 0.4 × 10(8) m(-1) s(-1) and 26 s(-1), respectively, were found to be consistent with the ΔF measurements.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

