The NF-κB1 transcription factor prevents the intrathymic development of CD8 T cells with memory properties
- PMID: 22124325
- PMCID: PMC3273395
- DOI: 10.1038/emboj.2011.435
The NF-κB1 transcription factor prevents the intrathymic development of CD8 T cells with memory properties
Abstract
The role of specific members of the NF-κB family of transcription factors in CD8 T-cell selection and development is largely unknown. Here, we show that mice lacking NF-κB1 develop a unique population of conventional CD8 single-positive (SP) thymocytes with memory T cell-like properties that populate peripheral immune organs. Development of this memory-like population is not due to PLZF(+) thymocytes and instead coincides with changes in CD8 T-cell selection. These include a reduction in the efficiency of negative selection and a dependence on MHC class Ia or Ib expressed by haematopoietic cells. These findings indicate that NF-κB1 regulates multiple events in the thymus that collectively inhibit the excess development of CD8(+) thymocytes with memory cell characteristics.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






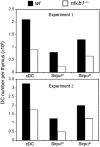
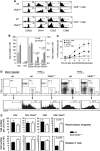
Similar articles
-
Bcl11b prevents the intrathymic development of innate CD8 T cells in a cell intrinsic manner.Int Immunol. 2015 Apr;27(4):205-15. doi: 10.1093/intimm/dxu104. Epub 2014 Nov 24. Int Immunol. 2015. PMID: 25422283
-
NF-kappa B is required for the positive selection of CD8+ thymocytes.J Immunol. 2000 Nov 1;165(9):5004-10. doi: 10.4049/jimmunol.165.9.5004. J Immunol. 2000. PMID: 11046028
-
The interferon regulatory transcription factor IRF-1 controls positive and negative selection of CD8+ thymocytes.Immunity. 1997 Aug;7(2):243-54. doi: 10.1016/s1074-7613(00)80527-0. Immunity. 1997. PMID: 9285409
-
Positive and negative thymocyte selection.Crit Rev Immunol. 1998;18(4):359-70. doi: 10.1615/critrevimmunol.v18.i4.40. Crit Rev Immunol. 1998. PMID: 9704194 Review.
-
[Positive and negative selection of thymocytes].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002 Oct;24(5):530-2. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002. PMID: 12905780 Review. Chinese.
Cited by
-
The histone methyltransferase DOT1L prevents antigen-independent differentiation and safeguards epigenetic identity of CD8+ T cells.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20706-20716. doi: 10.1073/pnas.1920372117. Epub 2020 Aug 6. Proc Natl Acad Sci U S A. 2020. PMID: 32764145 Free PMC article.
-
Dynamic aberrant NF-κB spurs tumorigenesis: a new model encompassing the microenvironment.Cytokine Growth Factor Rev. 2015 Aug;26(4):389-403. doi: 10.1016/j.cytogfr.2015.06.001. Epub 2015 Jun 20. Cytokine Growth Factor Rev. 2015. PMID: 26119834 Free PMC article. Review.
-
Signals for antigen-independent differentiation of memory CD8+ T cells.Cell Mol Life Sci. 2021 Oct;78(19-20):6395-6408. doi: 10.1007/s00018-021-03912-9. Epub 2021 Aug 16. Cell Mol Life Sci. 2021. PMID: 34398252 Free PMC article. Review.
-
Innate memory T cells.Adv Immunol. 2015;126:173-213. doi: 10.1016/bs.ai.2014.12.001. Epub 2015 Feb 7. Adv Immunol. 2015. PMID: 25727290 Free PMC article. Review.
-
Survival of Single Positive Thymocytes Depends upon Developmental Control of RIPK1 Kinase Signaling by the IKK Complex Independent of NF-κB.Immunity. 2019 Feb 19;50(2):348-361.e4. doi: 10.1016/j.immuni.2019.01.004. Epub 2019 Feb 5. Immunity. 2019. PMID: 30737145 Free PMC article.
References
-
- Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ (2006) The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 25: 79–91 - PubMed
-
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE (2001) Fine tuning of TCR signaling by CD5. J Immunol 166: 5464–5472 - PubMed
-
- Baldwin TA, Hogquist KA (2007) Transcriptional analysis of clonal deletion in vivo. J Immunol 179: 837–844 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

