Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay
- PMID: 22124462
- PMCID: PMC3237666
- DOI: 10.2337/db11-0670
Distinguishing persistent insulin autoantibodies with differential risk: nonradioactive bivalent proinsulin/insulin autoantibody assay
Abstract
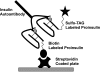
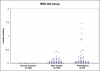
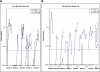
A subset of children develops persistent insulin autoantibodies (IAA; almost always as the only islet autoantibody) without evidence of progression to diabetes. The aim of the current study was the development and characterization of the performance of a nonradioactive fluid phase IAA assay in relation to standard IAA radioassay. We developed a nonradioactive IAA assay where bivalent IAA cross-link two insulin moieties in a fluid phase. The serum samples positive for anti-islet autoantibodies from 150 newly diagnosed patients with diabetes (Barbara Davis Center plus Diabetes Autoantibody Standardization Program [DASP] workshop) and 70 prediabetic subjects who were followed to diabetes were studied. In addition, sequential samples from 64 nondiabetic subjects who were persistently IAA(+) were analyzed. With 99th percentile of specificity, the new assay with the technology from Meso Scale Discovery Company (MSD-IAA) detects as positive 61% (61 of 100) of new-onset patients and 80% (56 of 70) of prediabetic patients compared with our current fluid phase micro-IAA radioassay (mIAA; 44 and 74%, respectively). In addition, MSD-IAA demonstrated better sensitivity than our mIAA from blinded DASP workshop (68 vs. 56% with the same 99% specificity). Of 64 IAA(+) nondiabetic subjects, 25% (8 of 32) who had only IAA and thus the low risk for progression to diabetes were positive with MSD-IAA assay. In contrast, 100% (32 of 32) high-risk children (IAA plus other islet autoantibodies) were positive with MSD-IAA. The IAA detectable by radioassay, but not MSD-IAA, were usually of lower affinity compared with the IAA of the high-risk children. These data suggest that a subset of IAA with current radioassay (not MSD-IAA) represents biologic false positives in terms of autoimmunity leading to diabetes. We hypothesize that factors related to the mechanism of loss of tolerance leading to diabetes determine high affinity and MSD-IAA reactivity.
Figures




Similar articles
-
Proinsulin/Insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity.Diabetes Care. 2013 Aug;36(8):2266-70. doi: 10.2337/dc12-2245. Epub 2013 Feb 19. Diabetes Care. 2013. PMID: 23423694 Free PMC article.
-
A novel LIPS assay for insulin autoantibodies.Acta Diabetol. 2018 Mar;55(3):263-270. doi: 10.1007/s00592-017-1082-y. Epub 2018 Jan 5. Acta Diabetol. 2018. PMID: 29305766
-
Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies.Diabetologia. 2010 Dec;53(12):2611-20. doi: 10.1007/s00125-010-1915-5. Epub 2010 Sep 25. Diabetologia. 2010. PMID: 20871974
-
International Workshop on Lessons From Animal Models for Human Type 1 Diabetes: identification of insulin but not glutamic acid decarboxylase or IA-2 as specific autoantigens of humoral autoimmunity in nonobese diabetic mice.Diabetes. 2001 Nov;50(11):2451-8. doi: 10.2337/diabetes.50.11.2451. Diabetes. 2001. PMID: 11679421 Review.
-
Developments in the prediction of type 1 diabetes mellitus, with special reference to insulin autoantibodies.Diabetes Metab Res Rev. 2005 Sep-Oct;21(5):395-415. doi: 10.1002/dmrr.554. Diabetes Metab Res Rev. 2005. PMID: 15895384 Review.
Cited by
-
Novel genetic risk factors influence progression of islet autoimmunity to type 1 diabetes.Sci Rep. 2020 Nov 5;10(1):19193. doi: 10.1038/s41598-020-75690-6. Sci Rep. 2020. PMID: 33154504 Free PMC article.
-
Predictors of slow progression to diabetes in children with multiple islet autoantibodies.J Autoimmun. 2016 Aug;72:113-7. doi: 10.1016/j.jaut.2016.05.010. Epub 2016 May 30. J Autoimmun. 2016. PMID: 27255734 Free PMC article.
-
Novel diabetes autoantibodies and prediction of type 1 diabetes.Curr Diab Rep. 2013 Oct;13(5):608-15. doi: 10.1007/s11892-013-0405-9. Curr Diab Rep. 2013. PMID: 23900975 Free PMC article. Review.
-
Islet Autoantibody Detection by Electrochemiluminescence (ECL) Assay.Methods Mol Biol. 2016;1433:85-91. doi: 10.1007/7651_2015_296. Methods Mol Biol. 2016. PMID: 26659802
-
Reliable diagnosis of murine type 1 diabetes using a panel of autoantigens and "antigen surrogates" mounted onto a liquid array.Mol Biosyst. 2015 Nov;11(11):3156-63. doi: 10.1039/c5mb00521c. Mol Biosyst. 2015. PMID: 26390856 Free PMC article.
References
-
- Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM. A novel micro-assay for insulin autoantibodies. J Autoimmun 1997;10:473–478 - PubMed
-
- Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ; Participating Laboratories Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 - PubMed
-
- Greenbaum CJ, Palmer JP, Kuglin B, Kolb H. Insulin autoantibodies measured by radioimmunoassay methodology are more related to insulin-dependent diabetes mellitus than those measured by enzyme-linked immunosorbent assay: results of the Fourth International Workshop on the Standardization of Insulin Autoantibody Measurement. J Clin Endocrinol Metab 1992;74:1040–1044 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

