Glucocorticoid-induced leucine zipper (GILZ) over-expression in T lymphocytes inhibits inflammation and tissue damage in spinal cord injury
- PMID: 22125095
- PMCID: PMC3271152
- DOI: 10.1007/s13311-011-0084-7
Glucocorticoid-induced leucine zipper (GILZ) over-expression in T lymphocytes inhibits inflammation and tissue damage in spinal cord injury
Abstract
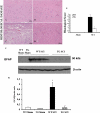
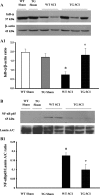
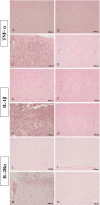
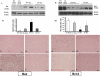
Spinal cord injury (SCI) is a traumatic event that causes a secondary and extended inflammation characterized by infiltration of immune cells, including T lymphocytes, release of pro-inflammatory mediators in the lesion site, and tissue degeneration. Current therapeutic approaches for SCI are limited to glucocorticoids (GC) due to their potent anti-inflammatory activity. GC efficacy resides, in part, in the capability to inhibit NF-κB, T lymphocyte activation, and the consequent cytokine production. In this study, we performed experiments aimed to test the susceptibility of glucocorticoid-induced leucine zipper (GILZ) transgenic (GILZ(TG)) mice, in which GILZ is selectively over-expressed in T lymphocytes, to SCI induction. Consistent with a decreased inflammatory response, GILZ(TG) were less susceptible to SCI as compared to wild-type littermates. Notably, inhibition of NF-κB activation and nuclear translocation, diminished T lymphocytes activation and tissue infiltration, as well as decreased release of cytokines were evident in GILZ(TG) as compared to wild-type mice. Moreover, GILZ(TG) showed a reduced tumor necrosis factor-α, IL-1β, Inductible nitric oxide synthase (iNOS) and nytrotyrosine production, apoptosis, and neuronal tissue damage. Together these results indicate that GILZ mimics the anti-inflammatory effect of GC and represents a potential pharmacological target for modulation of T lymphocyte-mediated immune response in inflammatory disorders, such as SCI.
Figures



Similar articles
-
Glucocorticoid-induced leucine zipper (GILZ) controls inflammation and tissue damage after spinal cord injury.CNS Neurosci Ther. 2014 Nov;20(11):973-81. doi: 10.1111/cns.12315. Epub 2014 Aug 21. CNS Neurosci Ther. 2014. PMID: 25146427 Free PMC article.
-
Glucocorticoid-induced leucine zipper is protective in Th1-mediated models of colitis.Gastroenterology. 2009 Feb;136(2):530-41. doi: 10.1053/j.gastro.2008.09.024. Epub 2008 Sep 25. Gastroenterology. 2009. PMID: 18996377
-
Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-κB pathway inhibition.J Neuroinflammation. 2019 Jan 17;16(1):12. doi: 10.1186/s12974-019-1394-7. J Neuroinflammation. 2019. PMID: 30654804 Free PMC article.
-
Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation.Front Immunol. 2019 Aug 7;10:1823. doi: 10.3389/fimmu.2019.01823. eCollection 2019. Front Immunol. 2019. PMID: 31440237 Free PMC article. Review.
-
GILZ as a Regulator of Cell Fate and Inflammation.Cells. 2021 Dec 30;11(1):122. doi: 10.3390/cells11010122. Cells. 2021. PMID: 35011684 Free PMC article. Review.
Cited by
-
Dynamic RBM47 ISGylation confers broad immunoprotection against lung injury and tumorigenesis via TSC22D3 downregulation.Cell Death Discov. 2023 Nov 30;9(1):430. doi: 10.1038/s41420-023-01736-z. Cell Death Discov. 2023. PMID: 38036512 Free PMC article.
-
Glucocorticoid-Induced Leucine Zipper (GILZ) in Cardiovascular Health and Disease.Cells. 2021 Aug 21;10(8):2155. doi: 10.3390/cells10082155. Cells. 2021. PMID: 34440924 Free PMC article. Review.
-
GILZ as a Mediator of the Anti-Inflammatory Effects of Glucocorticoids.Front Endocrinol (Lausanne). 2015 Nov 9;6:170. doi: 10.3389/fendo.2015.00170. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26617572 Free PMC article. Review.
-
Glucocorticoid-induced leucine zipper regulates liver fibrosis by suppressing CCL2-mediated leukocyte recruitment.Cell Death Dis. 2021 Apr 29;12(5):421. doi: 10.1038/s41419-021-03704-w. Cell Death Dis. 2021. PMID: 33927191 Free PMC article.
-
Glucocorticoid-induced leucine zipper (GILZ) is involved in glucocorticoid-induced and mineralocorticoid-induced leptin production by osteoarthritis synovial fibroblasts.Arthritis Res Ther. 2016 Oct 4;18(1):219. doi: 10.1186/s13075-016-1119-6. Arthritis Res Ther. 2016. PMID: 27716396 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

