Transethnic meta-analysis of genomewide association studies
- PMID: 22125221
- PMCID: PMC3460225
- DOI: 10.1002/gepi.20630
Transethnic meta-analysis of genomewide association studies
Abstract
The detection of loci contributing effects to complex human traits, and their subsequent fine-mapping for the location of causal variants, remains a considerable challenge for the genetics research community. Meta-analyses of genomewide association studies, primarily ascertained from European-descent populations, have made considerable advances in our understanding of complex trait genetics, although much of their heritability is still unexplained. With the increasing availability of genomewide association data from diverse populations, transethnic meta-analysis may offer an exciting opportunity to increase the power to detect novel complex trait loci and to improve the resolution of fine-mapping of causal variants by leveraging differences in local linkage disequilibrium structure between ethnic groups. However, we might also expect there to be substantial genetic heterogeneity between diverse populations, both in terms of the spectrum of causal variants and their allelic effects, which cannot easily be accommodated through traditional approaches to meta-analysis. In order to address this challenge, I propose novel transethnic meta-analysis methodology that takes account of the expected similarity in allelic effects between the most closely related populations, while allowing for heterogeneity between more diverse ethnic groups. This approach yields substantial improvements in performance, compared to fixed-effects meta-analysis, both in terms of power to detect association, and localization of the causal variant, over a range of models of heterogeneity between ethnic groups. Furthermore, when the similarity in allelic effects between populations is well captured by their relatedness, this approach has increased power and mapping resolution over random-effects meta-analysis.
© 2011 Wiley Periodicals, Inc.
Figures


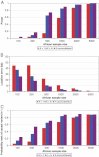
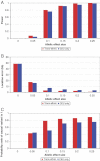
References
-
- Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, Yuan JM, Zheng W, Dawsey SM, Dong LM, Lee MP, Ding T, Qiao YL, Gao YT, Koh WP, Xiang YB, Tang ZZ, Fan JH, Wang C, Wheeler W, Gail MH, Yeager M, Yuenger J, Hutchinson A, Jacobs KB, Giffen CA, Burdett L, Fraumeni JF, Jr, Tucker MA, Chow WH, Goldstein AM, Chanock SJ, Taylor PR. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophagael squanous cell carcinoma. Nat Genet. 2010;42:764–767. - PMC - PubMed
-
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS Type 1 Diabetes Genetics Consortium. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. - PMC - PubMed
-
- Chambers JC, Zhao J, Terracciano CM, Bezzina CR, Zhang W, Kaba R, Navaratnarajah M, Lotlikar A, Sehmi JS, Kooner MK, Deng G, Siedlecka U, Parasramka S, El-Hamamsy I, Wass MN, Dekker LR, de Jong JS, Sternberg MJ, McKenna W, Severs NJ, de Silva R, Wilde AA, Anand P, Yacoub M, Scott J, Elliott P, Wood JN, Kooner JS. Genetic variation in SCN10A influences cardiac conduction. Nat Genet. 2010;42:149–152. - PubMed
-
- Chen ZJ, Zhao H, He L, Shi Y, Qin Y, Shi Y, Li Z, You L, Zhao J, Liu J, Liang X, Zhao X, Zhao J, Sun Y, Zhang B, Jiang H, Zhao D, Bian Y, Gao X, Geng L, Li Y, Zhu D, Sun X, Xu JE, Hao C, Ren CE, Zhang Y, Chen S, Zhang W, Yang A, Yan J, Li Y, Ma J, Zhao Y. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

