Evidence for water structuring forces between surfaces
- PMID: 22125414
- PMCID: PMC3223916
- DOI: 10.1016/j.cocis.2011.04.010
Evidence for water structuring forces between surfaces
Abstract
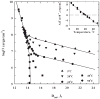
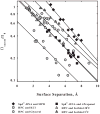
Structured water on apposing surfaces can generate significant energies due to reorganization and displacement of water as the surfaces encounter each other. Force measurements on a multitude of biological structures using the osmotic stress technique have elucidated commonalities that point toward an underlying hydration force. In this review, the forces of two contrasting systems are considered in detail: highly charged DNA and nonpolar, uncharged hydroxypropyl cellulose. Conditions for both net repulsion and attraction, along with the measured exclusion of chemically different solutes from these macromolecular surfaces, are explored and demonstrate common features consistent with a hydration force origin. Specifically, the observed interaction forces can be reduced to the effects of perturbing structured surface water.
Figures




Similar articles
-
Hydration forces underlie the exclusion of salts and of neutral polar solutes from hydroxypropylcellulose.J Phys Chem B. 2005 May 12;109(18):9111-8. doi: 10.1021/jp046999k. J Phys Chem B. 2005. PMID: 16852084
-
Water Structuring Induces Nonuniversal Hydration Repulsion between Polar Surfaces: Quantitative Comparison between Molecular Simulations, Theory, and Experiments.Langmuir. 2024 Apr 16;40(15):7896-7906. doi: 10.1021/acs.langmuir.3c03656. Epub 2024 Apr 5. Langmuir. 2024. PMID: 38578930 Free PMC article.
-
Anion Layering and Steric Hydration Repulsion on Positively Charged Surfaces in Aqueous Electrolytes.Chemphyschem. 2017 Nov 3;18(21):3056-3065. doi: 10.1002/cphc.201700865. Epub 2017 Oct 16. Chemphyschem. 2017. PMID: 28872763
-
Unraveling water's entropic mysteries: a unified view of nonpolar, polar, and ionic hydration.Acc Chem Res. 2008 Aug;41(8):957-67. doi: 10.1021/ar7001478. Acc Chem Res. 2008. PMID: 18710198 Review.
-
Measuring forces with the AFM: polymeric surfaces in liquids.Adv Colloid Interface Sci. 2002 Sep 16;99(1):13-75. doi: 10.1016/s0001-8686(02)00003-9. Adv Colloid Interface Sci. 2002. PMID: 12405400 Review.
Cited by
-
DNA bending-induced phase transition of encapsidated genome in phage λ.Nucleic Acids Res. 2013 Apr;41(8):4518-24. doi: 10.1093/nar/gkt137. Epub 2013 Feb 28. Nucleic Acids Res. 2013. PMID: 23449219 Free PMC article.
-
Influence of Microscopic Interactions on the Flexible Mechanical Properties of Viral DNA.Biophys J. 2018 Sep 4;115(5):763-772. doi: 10.1016/j.bpj.2018.07.023. Epub 2018 Aug 4. Biophys J. 2018. PMID: 30119833 Free PMC article.
-
Role of amino acid insertions on intermolecular forces between arginine peptide condensed DNA helices: implications for protamine-DNA packaging in sperm.J Biol Chem. 2011 Dec 9;286(49):41985-41992. doi: 10.1074/jbc.M111.295808. Epub 2011 Oct 12. J Biol Chem. 2011. PMID: 21994948 Free PMC article.
-
Hydration Repulsion between Carbohydrate Surfaces Mediated by Temperature and Specific Ions.Sci Rep. 2016 Jun 23;6:28553. doi: 10.1038/srep28553. Sci Rep. 2016. PMID: 27334145 Free PMC article.
-
Comparison of Sulfamethoxazole Removal Efficiency Using Polyethersulfone Ultrafiltration Membrane Modified by Various Methods.Materials (Basel). 2024 Dec 20;17(24):6247. doi: 10.3390/ma17246247. Materials (Basel). 2024. PMID: 39769846 Free PMC article.
References
-
- Ball P. Water as an active constituent in cell biology. Chem Rev. 2008;108:74–108. - PubMed
-
- McFearin CL, Beaman DK, Moore FG, Richmond GL. From Franklin to today: Toward a molecular level understanding of bonding and adsorption at the oil-water interface. J Phys Chem C. 2009;113:1171–88.
-
- Rasaiah JC, Garde S, Hummer G. Water in nonpolar confinement: From nanotubes to proteins and beyond. Annu Rev Phys Chem. 2008;59:713–40. - PubMed
-
- Park S, Moilanen DE, Fayer MD. Water dynamics - The effects of ions and nanoconfinement. J Phys Chem B. 2008;112:5279–90. - PubMed
-
- Mancinelli R, Imberti S, Soper AK, Liu KH, Mou CY, Bruni F, Ricci MA. Multiscale approach to the structural study of water confined in MCM41. J Phys Chem B. 2009;113:16169–77. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
