Protein disulfide isomerase and host-pathogen interaction
- PMID: 22125433
- PMCID: PMC3201685
- DOI: 10.1100/2011/289182
Protein disulfide isomerase and host-pathogen interaction
Abstract
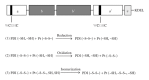
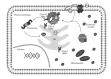
Reactive oxygen species (ROS) production by immunological cells is known to cause damage to pathogens. Increasing evidence accumulated in the last decade has shown, however, that ROS (and redox signals) functionally regulate different cellular pathways in the host-pathogen interaction. These especially affect (i) pathogen entry through protein redox switches and redox modification (i.e., intra- and interdisulfide and cysteine oxidation) and (ii) phagocytic ROS production via Nox family NADPH oxidase enzyme and the control of phagolysosome function with key implications for antigen processing. The protein disulfide isomerase (PDI) family of redox chaperones is closely involved in both processes and is also implicated in protein unfolding and trafficking across the endoplasmic reticulum (ER) and towards the cytosol, a thiol-based redox locus for antigen processing. Here, we summarise examples of the cellular association of host PDI with different pathogens and explore the possible roles of pathogen PDIs in infection. A better understanding of these complex regulatory steps will provide insightful information on the redox role and coevolutional biological process, and assist the development of more specific therapeutic strategies in pathogen-mediated infections.
Keywords: Host; Nox; PDI; endoplasmic reticulum; parasites; pathogen; redox.
Figures
Similar articles
-
Nox NADPH oxidases and the endoplasmic reticulum.Antioxid Redox Signal. 2014 Jun 10;20(17):2755-75. doi: 10.1089/ars.2013.5605. Epub 2014 Feb 26. Antioxid Redox Signal. 2014. PMID: 24386930 Free PMC article. Review.
-
Protein disulfide isomerases: Redox connections in and out of the endoplasmic reticulum.Arch Biochem Biophys. 2017 Mar 1;617:106-119. doi: 10.1016/j.abb.2016.11.007. Epub 2016 Nov 24. Arch Biochem Biophys. 2017. PMID: 27889386 Review.
-
Endoplasmic reticulum-dependent redox reactions control endoplasmic reticulum-associated degradation and pathogen entry.Antioxid Redox Signal. 2012 Apr 15;16(8):809-18. doi: 10.1089/ars.2011.4425. Epub 2012 Jan 30. Antioxid Redox Signal. 2012. PMID: 22142231 Free PMC article. Review.
-
Protein disulfide isomerase and Nox: new partners in redox signaling.Curr Pharm Des. 2015;21(41):5951-63. doi: 10.2174/1381612821666151029112523. Curr Pharm Des. 2015. PMID: 26510433 Review.
-
Protein disulfide isomerase in redox cell signaling and homeostasis.Free Radic Biol Med. 2012 May 1;52(9):1954-69. doi: 10.1016/j.freeradbiomed.2012.02.037. Epub 2012 Mar 6. Free Radic Biol Med. 2012. PMID: 22401853 Review.
Cited by
-
Novel roles for protein disulphide isomerase in disease states: a double edged sword?Front Cell Dev Biol. 2015 May 21;3:30. doi: 10.3389/fcell.2015.00030. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26052512 Free PMC article. Review.
-
A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19.Eur J Pharmacol. 2021 Jan 15;891:173748. doi: 10.1016/j.ejphar.2020.173748. Epub 2020 Nov 20. Eur J Pharmacol. 2021. PMID: 33227285 Free PMC article. Review.
-
Nox NADPH oxidases and the endoplasmic reticulum.Antioxid Redox Signal. 2014 Jun 10;20(17):2755-75. doi: 10.1089/ars.2013.5605. Epub 2014 Feb 26. Antioxid Redox Signal. 2014. PMID: 24386930 Free PMC article. Review.
-
The protein disulfide isomerase 1 of Phytophthora parasitica (PpPDI1) is associated with the haustoria-like structures and contributes to plant infection.Front Plant Sci. 2015 Aug 18;6:632. doi: 10.3389/fpls.2015.00632. eCollection 2015. Front Plant Sci. 2015. PMID: 26347756 Free PMC article.
-
Planthopper-Secreted Salivary Disulfide Isomerase Activates Immune Responses in Plants.Front Plant Sci. 2021 Jan 18;11:622513. doi: 10.3389/fpls.2020.622513. eCollection 2020. Front Plant Sci. 2021. PMID: 33537052 Free PMC article.
References
-
- Fenouillet E, Barbouche R, Jones IM. Cell entry by enveloped viruses: redox considerations for HIV and SARS-coronavirus. Antioxidants & Redox Signaling. 2007;9(8):1009–1034. - PubMed
-
- Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Current Opinion in Immunology. 1991;3(1):65–70. - PubMed
-
- Augusto O, Bonini MG, Amanso AM, Linares E, Santos CCX, De Menezes SL. Nitrogen dioxide and carbonate radical anion: two emerging radicals in biology. Free Radical Biology and Medicine. 2002;32(9):841–859. - PubMed
-
- Appenzeller-Herzog C, Ellgaard L. The human PDI family: versatility packed into a single fold. Biochimica et Biophysica Acta. 2008;1783(4):535–548. - PubMed
-
- Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxidants & Redox Signaling. 2009;11(11):2807–2850. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

