Functions of GSK-3 Signaling in Development of the Nervous System
- PMID: 22125510
- PMCID: PMC3221276
- DOI: 10.3389/fnmol.2011.00044
Functions of GSK-3 Signaling in Development of the Nervous System
Abstract
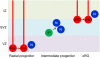
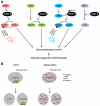
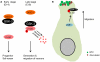
Glycogen synthase kinase-3 (GSK-3) is central to multiple intracellular pathways including those activated by Wnt/β-catenin, Sonic Hedgehog, Notch, growth factor/RTK, and G protein-coupled receptor signals. All of these signals importantly contribute to neural development. Early attention on GSK-3 signaling in neural development centered on the regulation of neuronal polarity using in vitro paradigms. However, recent creation of appropriate genetic models has demonstrated the importance of GSK-3 to multiple aspects of neural development including neural progenitor self-renewal, neurogenesis, neuronal migration, neural differentiation, and synaptic development.
Keywords: GSK-3; neural progenitor; neuronal migration.
Figures
Similar articles
-
Glycogen Synthase Kinase-3β Regulates Equilibrium Between Neurogenesis and Gliogenesis in Rat Model of Parkinson's Disease: a Crosstalk with Wnt and Notch Signaling.Mol Neurobiol. 2018 Aug;55(8):6500-6517. doi: 10.1007/s12035-017-0860-4. Epub 2018 Jan 11. Mol Neurobiol. 2018. PMID: 29327199
-
Effects of mutations in Wnt/β-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects on cell growth, metabolism and cancer.Biochim Biophys Acta. 2016 Dec;1863(12):2942-2976. doi: 10.1016/j.bbamcr.2016.09.004. Epub 2016 Sep 6. Biochim Biophys Acta. 2016. PMID: 27612668 Review.
-
Glycogen synthase kinase-3 inhibition promotes proliferation and neuronal differentiation of human-induced pluripotent stem cell-derived neural progenitors.Stem Cells Dev. 2012 Nov 20;21(17):3233-43. doi: 10.1089/scd.2011.0678. Epub 2012 Aug 10. Stem Cells Dev. 2012. PMID: 22642687
-
G protein coupled receptor 50 promotes self-renewal and neuronal differentiation of embryonic neural progenitor cells through regulation of notch and wnt/β-catenin signalings.Biochem Biophys Res Commun. 2015 Mar 20;458(4):836-42. doi: 10.1016/j.bbrc.2015.02.040. Epub 2015 Feb 14. Biochem Biophys Res Commun. 2015. PMID: 25689717
-
Glycogen synthase kinases: Moonlighting proteins with theranostic potential in cancer.Semin Cancer Biol. 2019 Jun;56:25-36. doi: 10.1016/j.semcancer.2017.12.010. Epub 2018 Jan 5. Semin Cancer Biol. 2019. PMID: 29309927 Review.
Cited by
-
GSK3β inhibition promotes synaptogenesis in Drosophila and mammalian neurons.PLoS One. 2015 Mar 12;10(3):e0118475. doi: 10.1371/journal.pone.0118475. eCollection 2015. PLoS One. 2015. PMID: 25764078 Free PMC article.
-
Neurodegenerative Disorder Risk in Krabbe Disease Carriers.Int J Mol Sci. 2022 Nov 4;23(21):13537. doi: 10.3390/ijms232113537. Int J Mol Sci. 2022. PMID: 36362324 Free PMC article.
-
The genetics of bipolar disorder with obesity and type 2 diabetes.J Affect Disord. 2022 Sep 15;313:222-231. doi: 10.1016/j.jad.2022.06.084. Epub 2022 Jun 30. J Affect Disord. 2022. PMID: 35780966 Free PMC article. Review.
-
Chronic N-Acetylcysteine Treatment Prevents Amphetamine-Induced Hyperactivity in Heterozygous Disc1 Mutant Mice, a Putative Prodromal Schizophrenia Animal Model.Int J Mol Sci. 2022 Aug 20;23(16):9419. doi: 10.3390/ijms23169419. Int J Mol Sci. 2022. PMID: 36012679 Free PMC article.
-
Microglia Polarization, Gene-Environment Interactions and Wnt/β-Catenin Signaling: Emerging Roles of Glia-Neuron and Glia-Stem/Neuroprogenitor Crosstalk for Dopaminergic Neurorestoration in Aged Parkinsonian Brain.Front Aging Neurosci. 2018 Feb 12;10:12. doi: 10.3389/fnagi.2018.00012. eCollection 2018. Front Aging Neurosci. 2018. PMID: 29483868 Free PMC article. Review.
References
-
- Akhmanova A., Hoogenraad C. C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B. M., De Zeeuw C. I., Grosveld F., Galjart N. (2001). Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104, 923–93510.1016/S0092-8674(01)00288-4 - DOI - PubMed
-
- Araki R., Hoki Y., Uda M., Nakamura M., Jincho Y., Tamura C., Sunayama M., Ando S., Sugiura M., Yoshida M. A., Kasama Y., Abe M. (2011). Crucial role of C-myc in the generation of induced pluripotent stem cells. Stem Cells 29, 1362–1370 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

