Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles
- PMID: 22125592
- PMCID: PMC3219638
- DOI: 10.1371/journal.pone.0025502
Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles
Abstract
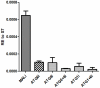
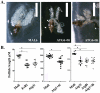
Autophagy plays a pivotal role by allowing cells to recycle cellular components under conditions of stress, starvation, development and cancer. In this work, we have demonstrated that programmed autophagy in the mosquito fat body plays a critical role in maintaining of developmental switches required for normal progression of gonadotrophic cycles. Mosquitoes must feed on vertebrate blood for their egg development, with each gonadotrophic cycle being tightly coupled to a separate blood meal. As a consequence, some mosquito species are vectors of pathogens that cause devastating diseases in humans and domestic animals, most importantly malaria and Dengue fever. Hence, deciphering mechanisms to control egg developmental cycles is of paramount importance for devising novel approaches for mosquito control. Central to egg development is vitellogenesis, the production of yolk protein precursors in the fat body, the tissue analogous to a vertebrate liver, and their subsequent specific accumulation in developing oocytes. During each egg developmental cycle, the fat body undergoes a developmental program that includes previtellogenic build-up of biosynthetic machinery, intense production of yolk protein precursors, and termination of vitellogenesis. The importance of autophagy for termination of vitellogenesis was confirmed by RNA interference (RNAi) depletions of several autophagic genes (ATGs), which inhibited autophagy and resulted in untimely hyper activation of TOR and prolonged production of the major yolk protein precursor, vitellogenin (Vg). RNAi depletion of the ecdysone receptor (EcR) demonstrated its activating role of autophagy. Depletion of the autophagic genes and of EcR led to inhibition of the competence factor, betaFTZ-F1, which is required for ecdysone-mediated developmental transitions. Moreover, autophagy-incompetent female mosquitoes were unable to complete the second reproductive cycle and exhibited retardation and abnormalities in egg maturation. Thus, our study has revealed a novel function of programmed autophagy in maintaining egg maturation cycles in mosquitoes.
Conflict of interest statement
Figures


Similar articles
-
A critical role of the nuclear receptor HR3 in regulation of gonadotrophic cycles of the mosquito Aedes aegypti.PLoS One. 2012;7(9):e45019. doi: 10.1371/journal.pone.0045019. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049766 Free PMC article.
-
The small GTPase Rheb is a key component linking amino acid signaling and TOR in the nutritional pathway that controls mosquito egg development.Insect Biochem Mol Biol. 2011 Jan;41(1):62-9. doi: 10.1016/j.ibmb.2010.10.001. Epub 2010 Oct 28. Insect Biochem Mol Biol. 2011. PMID: 21035549 Free PMC article.
-
microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti.Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22391-8. doi: 10.1073/pnas.1016230107. Epub 2010 Nov 29. Proc Natl Acad Sci U S A. 2010. PMID: 21115818 Free PMC article.
-
Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity.Insect Biochem Mol Biol. 2002 Oct;32(10):1275-86. doi: 10.1016/s0965-1748(02)00090-5. Insect Biochem Mol Biol. 2002. PMID: 12225918 Review.
-
Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny.Insect Biochem Mol Biol. 2005 Jul;35(7):661-75. doi: 10.1016/j.ibmb.2005.02.013. Epub 2005 Mar 28. Insect Biochem Mol Biol. 2005. PMID: 15894184 Review.
Cited by
-
20-Hydroxyecdysone upregulates Atg genes to induce autophagy in the Bombyx fat body.Autophagy. 2013 Aug;9(8):1172-87. doi: 10.4161/auto.24731. Epub 2013 May 14. Autophagy. 2013. PMID: 23674061 Free PMC article.
-
Interaction of Liberibacter Solanacearum with Host Psyllid Vitellogenin and Its Association with Autophagy.Microbiol Spectr. 2022 Aug 31;10(4):e0157722. doi: 10.1128/spectrum.01577-22. Epub 2022 Jul 11. Microbiol Spectr. 2022. PMID: 35863005 Free PMC article.
-
Eat to reproduce: a key role for the insulin signaling pathway in adult insects.Front Physiol. 2013 Aug 7;4:202. doi: 10.3389/fphys.2013.00202. eCollection 2013. Front Physiol. 2013. PMID: 23966944 Free PMC article.
-
Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality.Nat Commun. 2023 Sep 18;14(1):5773. doi: 10.1038/s41467-023-41370-y. Nat Commun. 2023. PMID: 37723154 Free PMC article.
-
Tyrosine transfer RNA levels and modifications during blood-feeding and vitellogenesis in the mosquito, Aedes aegypti.Insect Mol Biol. 2025 Feb;34(1):65-80. doi: 10.1111/imb.12950. Epub 2024 Aug 6. Insect Mol Biol. 2025. PMID: 39105593 Free PMC article.
References
-
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. - PubMed
-
- Tsukada M, Ohsumi Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993;333:169–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

