Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition
- PMID: 22125600
- PMCID: PMC3219632
- DOI: 10.1371/journal.pone.0026674
Suppressor of cytokine signalling-6 promotes neurite outgrowth via JAK2/STAT5-mediated signalling pathway, involving negative feedback inhibition
Abstract
Background: Suppressors of cytokine signalling (SOCS) protein family are key regulators of cellular responses to cytokines and play an important role in the nervous system. The SOCS6 protein, a less extensively studied SOCS family member, has been shown to induce insulin resistance in the retina and promote survival of the retinal neurons. But no reports are available about the role of SOCS6 in neuritogenesis. In this study, we examined the role of SOCS6 in neurite outgrowth and neuronal cell signalling.
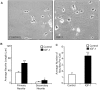
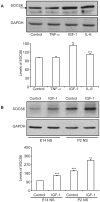
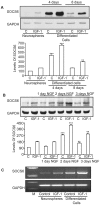
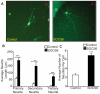
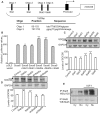
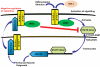
Methodology/principal findings: The effect of SOCS6 in neural stem cells differentiation was studied in neural stem cells and PC12 cell line. Highly elevated levels of SOCS6 were found upon neural cell differentiation both at the mRNA and protein level. Furthermore, SOCS6 over-expression lead to increase in neurite outgrowth and degree of branching, whereas SOCS6 knockdown with specific siRNAs, lead to a significant decrease in neurite initiation and extension. Insulin-like growth factor-1 (IGF-1) stimulation which enhanced neurite outgrowth of neural cells resulted in further enhancement of SOCS6 expression. Jak/Stat (Janus Kinase/Signal Transducer And Activator Of Transcription) pathway was found to be involved in the SOCS6 mediated neurite outgrowth. Bioinformatics study revealed presence of putative Stat binding sites in the SOCS6 promoter region. Transcription factors Stat5a and Stat5b were involved in SOCS6 gene upregulation leading to neuronal differentiation. Following differentiation, SOCS6 was found to form a ternary complex with IGFR (Insulin Like Growth Factor-1 Receptor) and JAK2 which acted in a negative feedback loop to inhibit pStat5 activation.
Conclusion/significance: The current paradigm for the first time states that SOCS6, a SOCS family member, plays an important role in the process of neuronal differentiation. These findings define a novel molecular mechanism for Jak2/Stat5 mediated SOCS6 signalling.
Conflict of interest statement
Figures





References
-
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. - PubMed
-
- Goldshmit Y, Greenhalgh CJ, Turnley AM. Suppressor of cytokine signalling-2 and epidermal growth factor regulate neurite outgrowth of cortical neurons. Eur J Neurosci. 2004;20:2260–22660. - PubMed
-
- Goldshmit Y, Walters CE, Scott HJ, Greenhalgh CJ, Turnley AM. SOCS2 induces neurite outgrowth by regulation of epidermal growth factor receptor activation. J Biol Chem. 2004;279:16349–16355. - PubMed
-
- Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signalling. J Cell Sci. 2000;113:2813–2819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

