Automated analysis of time-lapse imaging of nuclear translocation by retrospective strategy and its application to STAT1 in HeLa cells
- PMID: 22125613
- PMCID: PMC3220678
- DOI: 10.1371/journal.pone.0027454
Automated analysis of time-lapse imaging of nuclear translocation by retrospective strategy and its application to STAT1 in HeLa cells
Abstract
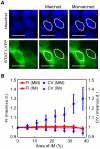
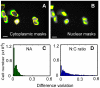
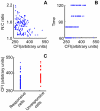
Cell-based image analysis of time-lapse imaging is mainly challenged by faint fluorescence and dim boundaries of cellular structures of interest. To resolve these bottlenecks, a novel method was developed based on "retrospective" analysis for cells undergoing minor morphological changes during time-lapse imaging. We fixed and stained the cells with a nuclear dye at the end of the experiment, and processed the time-lapse images using the binary masks obtained by segmenting the nuclear-stained image. This automated method also identifies cells that move during the time-lapse imaging, which is a factor that could influence the kinetics measured for target proteins that are present mostly in the cytoplasm. We then validated the method by measuring interferon gamma (IFNγ) induced signal transducers and activators of transcription 1 (STAT1) nuclear translocation in living HeLa cells. For the first time, automated large-scale analysis of nuclear translocation in living cells was achieved by our novel method. The responses of the cells to IFNγ exhibited a significant drift across the population, but common features of the responses led us to propose a three-stage model of STAT1 import. The simplicity and automation of this method should enable its application in a broad spectrum of time-lapse studies of nuclear-cytoplasmic translocation.
Conflict of interest statement
Figures




Similar articles
-
Live-cell measurements of kinase activity in single cells using translocation reporters.Nat Protoc. 2018 Jan;13(1):155-169. doi: 10.1038/nprot.2017.128. Epub 2017 Dec 21. Nat Protoc. 2018. PMID: 29266096
-
Green fluorescent protein-tagging reduces the nucleocytoplasmic shuttling specifically of unphosphorylated STAT1.FEBS J. 2007 Feb;274(3):815-26. doi: 10.1111/j.1742-4658.2006.05626.x. FEBS J. 2007. PMID: 17288561
-
Dynamic redistribution of STAT1 protein in IFN signaling visualized by GFP fusion proteins.Eur J Biochem. 1999 Feb;260(1):137-44. doi: 10.1046/j.1432-1327.1999.00149.x. Eur J Biochem. 1999. PMID: 10091593
-
STAT1 from the cell membrane to the DNA.EMBO J. 2001 May 15;20(10):2508-17. doi: 10.1093/emboj/20.10.2508. EMBO J. 2001. PMID: 11350940 Free PMC article.
-
Imaging of vaccinia virus entry into HeLa cells.Methods Mol Biol. 2012;890:123-33. doi: 10.1007/978-1-61779-876-4_7. Methods Mol Biol. 2012. PMID: 22688764
Cited by
-
Subcellular functions of proteins under fluorescence single-cell microscopy.Biochim Biophys Acta. 2016 Jan;1864(1):77-84. doi: 10.1016/j.bbapap.2015.05.014. Epub 2015 May 27. Biochim Biophys Acta. 2016. PMID: 26025769 Free PMC article. Review.
-
An Evolutionarily Conserved PLC-PKD-TFEB Pathway for Host Defense.Cell Rep. 2016 May 24;15(8):1728-42. doi: 10.1016/j.celrep.2016.04.052. Epub 2016 May 12. Cell Rep. 2016. PMID: 27184844 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

