Aging differentially affects multiple aspects of vesicle fusion kinetics
- PMID: 22125627
- PMCID: PMC3220683
- DOI: 10.1371/journal.pone.0027820
Aging differentially affects multiple aspects of vesicle fusion kinetics
Abstract
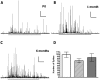
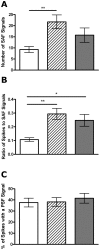
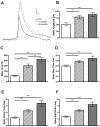
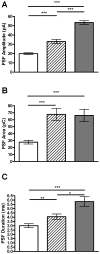
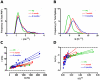
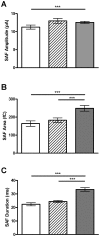
How fusion pore formation during exocytosis affects the subsequent release of vesicle contents remains incompletely understood. It is unclear if the amount released per vesicle is dependent upon the nature of the developing fusion pore and whether full fusion and transient kiss and run exocytosis are regulated by similar mechanisms. We hypothesise that if consistent relationships exist between these aspects of exocytosis then they will remain constant across any age. Using amperometry in mouse chromaffin cells we measured catecholamine efflux during single exocytotic events at P0, 1 month and 6 months. At all ages we observed full fusion (amperometric spike only), full fusion preceded by fusion pore flickering (pre-spike foot (PSF) signal followed by a spike) and pure "kiss and run" exocytosis (represented by stand alone foot (SAF) signals). We observe age-associated increases in the size of all 3 modes of fusion but these increases occur at different ages. The release probability of PSF signals or full spikes alone doesn't alter across any age in comparison with an age-dependent increase in the incidence of "kiss and run" type events. However, the most striking changes we observe are age-associated changes in the relationship between vesicle size and the membrane bending energy required for exocytosis. Our data illustrates that vesicle size does not regulate release probability, as has been suggested, that membrane elasticity or flexural rigidity change with age and that the mechanisms controlling full fusion may differ from those controlling "kiss and run" fusion.
Conflict of interest statement
Figures
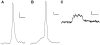
References
-
- Amatore C, Arbault S, Bonifas I, Guille M. Quantitative investigations of amperometric spike feet suggest different controlling factors of the fusion pore in exocytosis at chromaffin cells. Biophys Chem. 2009;143:124–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

