Nuclear lamina at the crossroads of the cytoplasm and nucleus
- PMID: 22126840
- PMCID: PMC3261324
- DOI: 10.1016/j.jsb.2011.11.007
Nuclear lamina at the crossroads of the cytoplasm and nucleus
Abstract
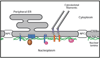
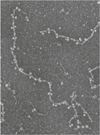
The nuclear lamina is a protein meshwork that lines the nuclear envelope in metazoan cells. It is composed largely of a polymeric assembly of lamins, which comprise a distinct sequence homology class of the intermediate filament protein family. On the basis of its structural properties, the lamina originally was proposed to provide scaffolding for the nuclear envelope and to promote anchoring of chromatin and nuclear pore complexes at the nuclear surface. This viewpoint has expanded greatly during the past 25 years, with a host of surprising new insights on lamina structure, molecular composition and functional attributes. It has been established that the self-assembly properties of lamins are very similar to those of cytoplasmic intermediate filament proteins, and that the lamin polymer is physically associated with components of the cytoplasmic cytoskeleton and with a multitude of chromatin and inner nuclear membrane proteins. Cumulative evidence points to an important role for the lamina in regulating signaling and gene activity, and in mechanically coupling the cytoplasmic cytoskeleton to the nucleus. The significance of the lamina has been vaulted to the forefront by the discovery that mutations in lamins and lamina-associated polypeptides lead to an array of human diseases. A key future challenge is to understand how the lamina integrates pathways for mechanics and signaling at the molecular level. Understanding the structure of the lamina from the atomic to supramolecular levels will be essential for achieving this goal.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. - PubMed
-
- Ben-Harush K, Wiesel N, Frenkiel-Krispin D, Moeller D, Soreq E, et al. The supramolecular organization of the C. elegans nuclear lamin filament. J Mol Biol. 2009;386:1392–1402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

