PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge
- PMID: 22126916
- PMCID: PMC3318143
- DOI: 10.1038/jcbfm.2011.167
PlGF knockout delays brain vessel growth and maturation upon systemic hypoxic challenge
Abstract
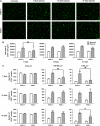
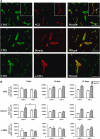
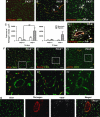
In this study, we have investigated the potential role of placental growth factor (PlGF) in hypoxia-induced brain angiogenesis. To this end, PlGF wild-type (PlGF(+/+)) and PlGF knockout (PlGF(-/-)) mice were exposed to whole body hypoxia (10% oxygen) for 7, 14, and 21 days. PlGF(+/+) animals exhibited a significant ~40% increase in angiogenesis after 7 days of hypoxia compared with controls, while in PlGF(-/-) this effect only occurred after 14 days of hypoxia. No differences in pericyte/smooth muscle cell (SMC) coverage between the two genotypes were observed. After 14 days of hypoxia, PlGF(-/-) microvessels had a significant increase in fibrinogen accumulation and extravasation compared with those of PlGF(+/+), which correlated with endothelial cell disruption of the tight junction protein claudin-5. These vessels displayed large lumens, were surrounded by reactive astrocytes, lacked both pericyte/SMC coverage and endothelial vascular endothelial growth factor expression, and regressed after 21 days of hypoxia. Vascular endothelial growth factor expression levels were found to be significantly lower in the frontal cortex of PlGF(-/-) compared with those in PlGF(+/+) animals during the first 5 days of hypoxia, which in combination with the lack of PlGF may have contributed to the delayed angiogenic response and the prothrombotic phenotype observed in the PlGF(-/-)animals.
Figures
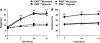


Similar articles
-
Stromal Claudin14-heterozygosity, but not deletion, increases tumour blood leakage without affecting tumour growth.PLoS One. 2013 May 13;8(5):e62516. doi: 10.1371/journal.pone.0062516. Print 2013. PLoS One. 2013. PMID: 23675413 Free PMC article.
-
Placenta growth factor and vascular endothelial growth factor B expression in the hypoxic lung.Respir Res. 2011 Jan 25;12(1):17. doi: 10.1186/1465-9921-12-17. Respir Res. 2011. PMID: 21266048 Free PMC article.
-
VEGFR-2-mediated increased proliferation and survival in response to oxygen and glucose deprivation in PlGF knockout astrocytes.J Neurochem. 2008 Nov;107(3):756-67. doi: 10.1111/j.1471-4159.2008.05660.x. Epub 2008 Sep 11. J Neurochem. 2008. PMID: 18786179
-
Roles of Hypoxia Response in Retinal Development and Pathophysiology.Keio J Med. 2018 Mar 23;67(1):1-9. doi: 10.2302/kjm.2017-0002-IR. Epub 2017 Jun 6. Keio J Med. 2018. PMID: 28592747 Review.
-
Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review.Placenta. 2000 Mar-Apr;21 Suppl A:S16-24. doi: 10.1053/plac.1999.0524. Placenta. 2000. PMID: 10831117 Review.
Cited by
-
Cognitive Dysfunction after Heart Disease: A Manifestation of the Heart-Brain Axis.Oxid Med Cell Longev. 2021 Aug 18;2021:4899688. doi: 10.1155/2021/4899688. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34457113 Free PMC article. Review.
-
Therapeutic potential of ixmyelocel-T, an expanded autologous multicellular therapy for treatment of ischemic cardiovascular diseases.Stem Cell Res Ther. 2015 Mar 13;6(1):25. doi: 10.1186/s13287-015-0007-3. Stem Cell Res Ther. 2015. PMID: 25889271 Free PMC article.
-
The Beneficial Potential of Genetically Modified Stem Cells in the Treatment of Stroke: a Review.Stem Cell Rev Rep. 2022 Feb;18(2):412-440. doi: 10.1007/s12015-021-10175-1. Epub 2021 May 25. Stem Cell Rev Rep. 2022. PMID: 34033001 Free PMC article. Review.
-
Genetic mouse models to study blood-brain barrier development and function.Fluids Barriers CNS. 2013 Jan 10;10(1):3. doi: 10.1186/2045-8118-10-3. Fluids Barriers CNS. 2013. PMID: 23305182 Free PMC article.
-
Vascular endothelial growth factors (VEGFs) and stroke.Cell Mol Life Sci. 2013 May;70(10):1753-61. doi: 10.1007/s00018-013-1282-8. Epub 2013 Mar 12. Cell Mol Life Sci. 2013. PMID: 23475070 Free PMC article. Review.
References
-
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. - PMC - PubMed
-
- Adini A, Kornaga T, Firoozbakht F, Benjamin LE. Placental growth factor is a survival factor for tumor endothelial cells and macrophages. Cancer Res. 2002;62:2749–2752. - PubMed
-
- Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen—a review. Placenta. 2000;21 (Suppl A:S16–S24. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. - PubMed
-
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood--brain barrier. Nature. 2010;468:557–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

