Bacterial-fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists
- PMID: 22126995
- PMCID: PMC3232736
- DOI: 10.1128/MMBR.00020-11
Bacterial-fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists
Abstract
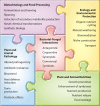
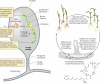
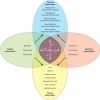
Bacteria and fungi can form a range of physical associations that depend on various modes of molecular communication for their development and functioning. These bacterial-fungal interactions often result in changes to the pathogenicity or the nutritional influence of one or both partners toward plants or animals (including humans). They can also result in unique contributions to biogeochemical cycles and biotechnological processes. Thus, the interactions between bacteria and fungi are of central importance to numerous biological questions in agriculture, forestry, environmental science, food production, and medicine. Here we present a structured review of bacterial-fungal interactions, illustrated by examples sourced from many diverse scientific fields. We consider the general and specific properties of these interactions, providing a global perspective across this emerging multidisciplinary research area. We show that in many cases, parallels can be drawn between different scenarios in which bacterial-fungal interactions are important. Finally, we discuss how new avenues of investigation may enhance our ability to combat, manipulate, or exploit bacterial-fungal complexes for the economic and practical benefit of humanity as well as reshape our current understanding of bacterial and fungal ecology.
Figures










References
-
- Adams A. S., Currie C. R., Cardoza Y., Klepzig K. D., Raffa K. F. 2009. Effects of symbiotic bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can. J. Forest Res. 39:1133–1147
-
- Addis E., Fleet G. H., Cox J. M., Kolak D., Leung T. 2001. The growth, properties and interactions of yeasts and bacteria associated with the maturation of Camembert and blue-veined cheeses. Int. J. Food Microbiol. 69:25–36 - PubMed
-
- Adesina M. F., Grosch R., Lembke A., Vatchev T. D., Smalla K. 2009. In vitro antagonists of Rhizoctonia solani tested on lettuce: rhizosphere competence, biocontrol efficiency and rhizosphere microbial community response. FEMS Microbiol. Ecol. 69:62–74 - PubMed
-
- Aho P. E., Seidler R. J., Evans H. J., Raju P. N. 1974. Distribution, enumeration, and identification of nitrogen-fixing bacteria associated with decay in living white fir trees. Phytopathology 64:1413–1420
-
- Alexandre H., Costello P. J., Remize F., Guzzo J., Guilloux-Benatier M. 2004. Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: current knowledge and perspectives. Int. J. Food Microbiol. 93:141–154 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

