Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review
- PMID: 22127225
- PMCID: PMC3418064
- DOI: 10.1016/j.actbio.2011.11.017
Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review
Abstract
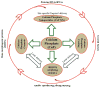
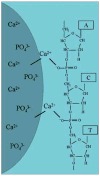
Calcium phosphates (CaPs) are the most widely used bone substitutes in bone tissue engineering due to their compositional similarities to bone mineral and excellent biocompatibility. In recent years, CaPs, especially hydroxyapatite and tricalcium phosphate, have attracted significant interest in simultaneous use as bone substitute and drug delivery vehicle, adding a new dimension to their application. CaPs are more biocompatible than many other ceramic and inorganic nanoparticles. Their biocompatibility and variable stoichiometry, thus surface charge density, functionality, and dissolution properties, make them suitable for both drug and growth factor delivery. CaP matrices and scaffolds have been reported to act as delivery vehicles for growth factors and drugs in bone tissue engineering. Local drug delivery in musculoskeletal disorder treatments can address some of the critical issues more effectively and efficiently than the systemic delivery. CaPs are used as coatings on metallic implants, CaP cements, and custom designed scaffolds to treat musculoskeletal disorders. This review highlights some of the current drug and growth factor delivery approaches and critical issues using CaP particles, coatings, cements, and scaffolds towards orthopedic and dental applications.
Copyright © 2011 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures








References
-
- National Osteoporosis Foundation. Available from: http://www.nof.org.
-
- Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997–2004. Arthritis Rheum. 2008;59(4):481–8. - PubMed
-
- American Academy Of Orthopaedic Surgeons (AAOS) Available from: http://orthoinfo.aaos.org.
-
- United States Bone and Joint Initiative. Available from: http://www.usbjd.org.
-
- Bose S, Tarafder S, Edgington J, Bandyopadhyay A. Calcium phosphate ceramics in drug delivery. JOM. 2011;63:93–8.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

