Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study
- PMID: 22127229
- PMCID: PMC3287353
- DOI: 10.1152/ajpendo.00290.2011
Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study
Abstract
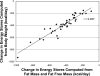
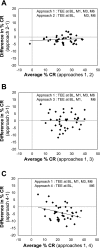
Calorie restriction (CR) is a component of most weight loss interventions and a potential strategy to slow aging. Accurate determination of energy intake and %CR is critical when interpreting the results of CR interventions; this is most accurately achieved using the doubly labeled water method to quantify total energy expenditure (TEE). However, the costs and analytical requirements of this method preclude its repeated use in many clinical trials. Our aims were to determine 1) the optimal TEE assessment time points for quantifying average energy intake and %CR during long-term CR interventions and 2) the optimal approach for quantifying short-term changes in body energy stores to determine energy intake and %CR during 2-wk DLW periods. Adults randomized to a CR intervention in the multicenter CALERIE study underwent measurements of TEE by doubly labeled water and body composition at baseline and months 1, 3, and 6. Average %CR achieved during the intervention was 24.9 ± 8.7%, which was computed using an approach that included four TEE assessment time points (i.e., TEE(baseline, months 1, 3, and 6)) plus the 6-mo change in body composition. Approaches that included fewer TEE assessments yielded %CR values of 23.4 ± 9.0 (TEE(baseline,) months 3 and 6), 25.0 ± 8.7 (TEE(baseline,) months 1 and 6), and 20.9 ± 7.1% (TEE(baseline, month 6)); the latter approach differed significantly from approach 1 (P < 0.001). TEE declined 9.6 ± 9.9% within 2-4 wk of CR beginning and then stabilized. Regression of daily home weights provided the most reliable estimate of short-term change in energy stores. In summary, optimal quantification of energy intake and %CR during weight loss necessitates a TEE measurement within the first month of CR to capture the rapid reduction in TEE.
Trial registration: ClinicalTrials.gov NCT00099099 NCT00099138 NCT00099151.
Figures


References
-
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 85: 1023–1030, 2007 - PubMed
-
- de Boer JO, van Es AJ, Roovers LC, van Raaij JM, Hautvast JG. Adaptation of energy metabolism of overweight women to low-energy intake, studied with whole-body calorimeters. Am J Clin Nutr 44: 585–595, 1986 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- M01-RR-00036/RR/NCRR NIH HHS/United States
- U01-AG022132/AG/NIA NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- U01-AG-020478/AG/NIA NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- U01-AG-020480/AG/NIA NIH HHS/United States
- U01-AG-020487/AG/NIA NIH HHS/United States
- R00 HD060762/HD/NICHD NIH HHS/United States
- P30-DK-056341/DK/NIDDK NIH HHS/United States
- P60-DK-020579-30/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

