Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium
- PMID: 22127232
- PMCID: PMC3265797
- DOI: 10.4161/chan.5.6.18523
Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium
Abstract
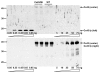
Gap junction channels in ventricular myocardium are required for electrical and metabolic coupling between cardiac myocytes and for normal cardiac pump function. Although much is known about expression patterns and remodeling of cardiac connexin(Cx)43, little is known about the less abundant Cx45, which is required for embryonic development and viability, is downregulated in adult hearts, and is pathophysiologically upregulated in human end-stage heart failure. We applied quantitative immunoblotting and immunoprecipitation to native myocardial extracts, immunogold electron microscopy to cardiac tissue and membrane sections, electrophysiological recordings to whole hearts, and high-resolution tandem mass spectrometry to Cx45 fusion protein, and developed two new tools, anti-Cx45 antisera and Cre(+);Cx45 floxed mice, to facilitate characterization of Cx45 in adult mammalian hearts. We found that Cx45 represents 0.3% of total Cx protein (predominantly 200 fmol Cx43 protein/μg ventricular protein) and colocalizes with Cx43 in native ventricular gap junctions, particularly in the apex and septum. Cre(+);Cx45 floxed mice express 85% less Cx45, but do not exhibit overt electrophysiologic abnormalities. Although the basal phosphorylation status of native Cx45 remains unknown, CaMKII phosphorylates 8 Ser/Thr residues in Cx45 in vitro. Thus, although downregulation of Cx45 does not produce notable deficits in electrical conduction in adult, disease-free hearts, Cx45 is a target of the multifunctional kinase CaMKII, and the phosphorylation status of Cx45 and the role of Cx43/Cx45 heteromeric gap junction channels in both normal and diseased hearts merits further investigation.
Figures





References
-
- Veenstra RD, Wang HZ, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994;75:483–490. - PubMed
-
- Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994;74:1050–1057. - PubMed
-
- Beblo DA, Wang HZ, Beyer EC, Westphale EM, Veestra RD. Unique conductance, gating and selective permeability properties of gap junction channels formed by connexin40. Circ Res. 1995;77:813–822. - PubMed
-
- Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
