The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission
- PMID: 22127301
- PMCID: PMC3645314
- DOI: 10.1038/nrn3138
The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission
Abstract
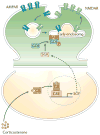
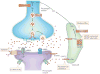
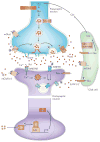
Mounting evidence suggests that acute and chronic stress, especially the stress-induced release of glucocorticoids, induces changes in glutamate neurotransmission in the prefrontal cortex and the hippocampus, thereby influencing some aspects of cognitive processing. In addition, dysfunction of glutamatergic neurotransmission is increasingly considered to be a core feature of stress-related mental illnesses. Recent studies have shed light on the mechanisms by which stress and glucocorticoids affect glutamate transmission, including effects on glutamate release, glutamate receptors and glutamate clearance and metabolism. This new understanding provides insights into normal brain functioning, as well as the pathophysiology and potential new treatments of stress-related neuropsychiatric disorders.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

