Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide metaflumizone
- PMID: 22127519
- PMCID: PMC3286292
- DOI: 10.1124/mol.111.075283
Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide metaflumizone
Abstract
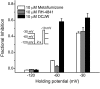
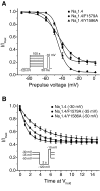
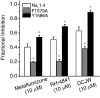
Sodium channel inhibitor (SCI) insecticides selectively target voltage-gated sodium (Na(v)) channels in the slow-inactivated state by binding at or near the local anesthetic receptor within the sodium channel pore. Metaflumizone is a new insecticide for the treatment of fleas on domesticated pets and has recently been reported to block insect sodium channels in the slow-inactivated state, thereby implying that it is also a member of the SCI class. Using the two-electrode voltage-clamp technique, we examined metaflumizone inhibition of rat Na(v)1.4 sodium channels expressed in Xenopus laevis oocytes. Metaflumizone selectively inhibited Na(v)1.4 channels at potentials that promoted slow inactivation and shifted the voltage dependence of slow inactivation in the direction of hyperpolarization. Metaflumizone perfusion at a hyperpolarized holding potential also shifted the conductance-voltage curve for activation in the direction of depolarization and antagonized use-dependent lidocaine inhibition of fast-inactivated sodium channels, actions not previously observed with other SCI insecticides. We expressed mutated Na(v)1.4/F1579A and Na(v)1.4/Y1586A channels to investigate whether metaflumizone shares the domain IV segment S6 (DIV-S6) binding determinants identified for other SCI insecticides. Consistent with previous investigations of SCI insecticides on rat Na(v)1.4 channels, the F1579A mutation reduced sensitivity to block by metaflumizone, whereas the Y1586A mutation paradoxically increased the sensitivity to metaflumizone. We conclude that metaflumizone selectively inhibits slow-inactivated Na(v)1.4 channels and shares DIV-S6 binding determinants with other SCI insecticides and therapeutic drugs. However, our results suggest that metaflumizone interacts with resting and fast-inactivated channels in a manner that is distinct from other compounds in this insecticide class.
Figures


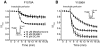
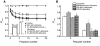
References
-
- Bai CX, Glaaser IW, Sawanobori T, Sunami A. (2003) Involvement of local anesthetic binding sites on IVS6 of sodium channels in fast and slow inactivation. Neurosci Lett 337:41–45 - PubMed
-
- Errington AC, Stöhr T, Heers C, Lees G. (2008) The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol 73:157–169 - PubMed
-
- Goldin AL. (1992) Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol 207:266–279 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

