Investigating the relative efficacies of combination chemotherapy of paclitaxel/carboplatin, with or without anthracycline, for endometrial carcinoma
- PMID: 22127553
- PMCID: PMC3325403
- DOI: 10.1007/s00404-011-2154-9
Investigating the relative efficacies of combination chemotherapy of paclitaxel/carboplatin, with or without anthracycline, for endometrial carcinoma
Abstract
Purpose: Recently a combination of paclitaxel and carboplatin (TC) (without an anthracycline) has begun to be used as an adjuvant or remission induction therapy, without any critical supportive evidence of its efficacy relative to a combination chemotherapy of taxane, platinum and anthracycline such as TEC (paclitaxel, epirubicin and carboplatin). The aim of our present study was to conduct the required clinical evaluations of the relative effectiveness of TC compared to TEC.
Methods: A retrospective comparison between the efficacy of TEC and TC regimens used for endometrial carcinoma at the Osaka University Hospital and the Osaka Medical Center for Cancer and Cardiovascular Diseases in Osaka, Japan, respectively, from 1999 to 2009 was performed. The clinical characteristics of the patients who received either TEC or TC were not significantly different, and TEC and TC therapies were initiated based on similar indications for chemotherapy. TEC regimen was paclitaxel (150 mg/m(2)), epirubicin (50 mg/m(2)) and carboplatin (AUC 4). TC regimen consisted of paclitaxel (175 mg/m(2)) and carboplatin (AUC 5).
Results: TEC was demonstrated to provide significantly better survival than TC as an adjuvant therapy for resected Stage III/IV diseases (p = 0.017 for progression-free survival and p = 0.014 for overall survival, by the log-rank test). However, in recurrent or more advanced cases, TC and TEC demonstrated similar effects on survival (p = 0.55 for progression-free survival and p = 0.63 for overall survival).
Conclusions: TEC should be offered as an adjuvant therapy to Stage III/IV patients. TC may be considered for recurrent or unresectable cases as a remission induction therapy.
Figures

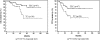
References
-
- DiSaia PJ, Creasman WT. Clinical Gynecologic Oncology. 6. St. Louis: Mosby; 2002.
-
- Berek JS. Novak’s Gynecology. 13. Baltimore: William and Wilkins; 2002.
-
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, Thigpen JT, Benda JA, Gynecologic Oncology Group Study Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. - DOI - PubMed
-
- Muss HB. Chemotherapy of metastatic endometrial cancer. Semin Oncol. 1994;21:107–113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

