Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics
- PMID: 22127588
- PMCID: PMC3254197
- DOI: 10.1093/jac/dkr483
Ascorbic acid protects against the nephrotoxicity and apoptosis caused by colistin and affects its pharmacokinetics
Abstract
Objectives: The use of colistin in the treatment of life-threatening Gram-negative infections is associated with a high rate of nephrotoxicity that is dose limiting. This study aimed to examine the nephroprotective effect of ascorbic acid against colistin-induced nephrotoxicity.
Methods: Rats were treated intravenously twice daily with saline, colistin (cumulative dose of 36.5 mg/kg), a combination of ascorbic acid (50 or 200 mg/kg) and colistin, or ascorbic acid (200 mg/kg) over 7 days. Colistin-induced apoptosis was examined in rats over 5 days and in vitro using rat renal proximal tubular cells NRK-52E over 24 h with and without ascorbic acid. The effect of co-administered ascorbic acid on colistin pharmacokinetics was investigated.
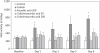
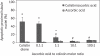
Results: The 24 h urinary excretion of N-acetyl-β-D-glucosaminidase, a sensitive marker for tubular damage, was significantly lower (P < 0.0001) in the colistin/ascorbic acid 200 mg/kg group. Significant histological abnormalities (P < 0.01) were detected only in the kidneys of the colistin group, which also had the highest percentage (30.6 ± 7.8%) of apoptotic cells (P < 0.005). In the cell culture studies, the percentage of apoptotic cells was significantly higher in the presence of 0.1 mM colistin alone (51.8 ± 2.0%; P < 0.0001) than in the presence of ascorbic acid, which decreased the apoptotic effect in a concentration-dependent manner. Ascorbic acid (200 mg/kg) altered colistin pharmacokinetics, as the total body clearance decreased from 3.78 ± 0.36 mL/min/kg (colistin group) to 2.46 ± 0.57 mL/min/kg (P = 0.0024).
Conclusions: This is the first study demonstrating the protective effect of ascorbic acid against colistin-induced nephrotoxicity and tubular apoptosis. Co-administration of ascorbic acid has the potential to increase the therapeutic index of colistin.
Figures



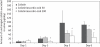
Similar articles
-
Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity.Antimicrob Agents Chemother. 2015;59(6):3224-32. doi: 10.1128/AAC.00280-15. Epub 2015 Mar 23. Antimicrob Agents Chemother. 2015. PMID: 25801556 Free PMC article. Clinical Trial.
-
Melatonin attenuates colistin-induced nephrotoxicity in rats.Antimicrob Agents Chemother. 2011 Sep;55(9):4044-9. doi: 10.1128/AAC.00328-11. Epub 2011 Jun 27. Antimicrob Agents Chemother. 2011. PMID: 21709095 Free PMC article.
-
Gelofusine Ameliorates Colistin-Induced Nephrotoxicity.Antimicrob Agents Chemother. 2017 Nov 22;61(12):e00985-17. doi: 10.1128/AAC.00985-17. Print 2017 Dec. Antimicrob Agents Chemother. 2017. PMID: 28923868 Free PMC article.
-
Prevention of colistin induced nephrotoxicity: a review of preclinical and clinical data.Expert Rev Clin Pharmacol. 2021 Sep;14(9):1113-1131. doi: 10.1080/17512433.2021.1933436. Epub 2021 Jun 9. Expert Rev Clin Pharmacol. 2021. PMID: 34015235 Review.
-
A review on colistin nephrotoxicity.Eur J Clin Pharmacol. 2015 Jul;71(7):801-10. doi: 10.1007/s00228-015-1865-4. Epub 2015 May 27. Eur J Clin Pharmacol. 2015. PMID: 26008213 Review.
Cited by
-
Structure, Function, and Biosynthetic Origin of Octapeptin Antibiotics Active against Extensively Drug-Resistant Gram-Negative Bacteria.Cell Chem Biol. 2018 Apr 19;25(4):380-391.e5. doi: 10.1016/j.chembiol.2018.01.005. Epub 2018 Feb 3. Cell Chem Biol. 2018. PMID: 29396290 Free PMC article.
-
Evaluation of Dose-Fractionated Polymyxin B on Acute Kidney Injury Using a Translational In Vivo Rat Model.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e02300-19. doi: 10.1128/AAC.02300-19. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32071049 Free PMC article.
-
International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP).Pharmacotherapy. 2019 Jan;39(1):10-39. doi: 10.1002/phar.2209. Pharmacotherapy. 2019. PMID: 30710469 Free PMC article.
-
Megalin contributes to kidney accumulation and nephrotoxicity of colistin.Antimicrob Agents Chemother. 2013 Dec;57(12):6319-24. doi: 10.1128/AAC.00254-13. Epub 2013 Oct 7. Antimicrob Agents Chemother. 2013. PMID: 24100504 Free PMC article.
-
The Role of Mitochondria in Drug-Induced Kidney Injury.Front Physiol. 2020 Sep 4;11:1079. doi: 10.3389/fphys.2020.01079. eCollection 2020. Front Physiol. 2020. PMID: 33013462 Free PMC article. Review.
References
-
- Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–7. doi:10.1345/aph.18426. - DOI - PubMed
-
- Falagas ME, Kasiakou SK, Falagas ME, et al. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40:1333–41. doi:10.1086/429323. - DOI - PubMed
-
- Landman D, Georgescu C, Martin DA, et al. Polymyxins revisited. Clin Microbiol Rev. 2008;21:449–65. doi:10.1128/CMR.00006-08. - DOI - PMC - PubMed
-
- Li J, Nation RL, Milne RW, et al. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi:10.1016/j.ijantimicag.2004.10.001. - DOI - PubMed
-
- Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi:10.1016/S1473-3099(06)70580-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

