Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites
- PMID: 22127611
- PMCID: PMC3382282
- DOI: 10.1007/s00894-011-1306-y
Quantum-chemical investigation of the structure and the antioxidant properties of α-lipoic acid and its metabolites
Abstract
Quantum-chemical computations were used to investigate the structure-antioxidant parameter relationships of α-lipoic acid and its natural metabolites bisnorlipoic acid and tetranorlipoic acid in their oxidized and reduced forms. The enantiomers of lipoic and dihydrolipoic acid were optimized using the B3LYP/6-311+G(3df,2p), B3LYP/aug-cc-pVDZ and MP2(full)/6-31+G(d,p) levels of theory as isolated molecules and in the presence of water. The geometries of the metabolites and the values of their antioxidant parameters (proton affinity, bond dissociation enthalpy, adiabatic ionization potential, spin density, and the highest occupied molecular orbital energy) were calculated at the B3LYP/6-311+G(3df,2p) level of theory. The results obtained reveal similarities between these structures: a pentatomic, nonaromatic ring is present in the oxidized forms, while an unbranched aliphatic chain (as found in saturated fatty acids) is present in both the oxidized and the reduced forms. Analysis of the spin density and the highest occupied molecular orbital energy revealed that the SH groups exhibited the greatest electron-donating activities. The values obtained for the proton affinity, bond dissociation enthalpy and adiabatic ionization potential indicate that the preferred antioxidant mechanisms for α-lipoic acid and its metabolites are sequential proton loss electron transfer in polar media and hydrogen atom transfer in vacuum.
Figures
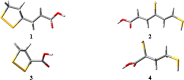
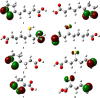
Similar articles
-
2D NMR of the metabolic antioxidant dihydrolipoic acid and its derivatives.Free Radic Res. 1996 Sep;25(3):195-205. doi: 10.3109/10715769609149045. Free Radic Res. 1996. PMID: 8889486
-
Redox homeostasis of albumin in relation to alpha-lipoic acid and dihydrolipoic acid.Oxid Med Cell Longev. 2010 May-Jun;3(3):206-13. doi: 10.4161/oxim.3.3.11786. Oxid Med Cell Longev. 2010. PMID: 20716945 Free PMC article.
-
Evaluation of the antioxidant capacity of ubiquinol and dihydrolipoic acid.Z Naturforsch C J Biosci. 1998 Mar-Apr;53(3-4):250-3. doi: 10.1515/znc-1998-3-415. Z Naturforsch C J Biosci. 1998. PMID: 9618938
-
Antioxidant and prooxidant activities of alpha-lipoic acid and dihydrolipoic acid.Toxicol Appl Pharmacol. 2002 Jul 1;182(1):84-90. doi: 10.1006/taap.2002.9437. Toxicol Appl Pharmacol. 2002. PMID: 12127266 Review.
-
Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention.Rev Bras Farmacogn. 2023;33(2):272-287. doi: 10.1007/s43450-023-00370-1. Epub 2023 Feb 7. Rev Bras Farmacogn. 2023. PMID: 36778891 Free PMC article. Review.
Cited by
-
Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids.Sci Rep. 2020 Feb 13;10(1):2611. doi: 10.1038/s41598-020-59451-z. Sci Rep. 2020. PMID: 32054964 Free PMC article.
-
Density Functional Theory Studies on the Chemical Reactivity of Allyl Mercaptan and Its Derivatives.Molecules. 2024 Jan 31;29(3):668. doi: 10.3390/molecules29030668. Molecules. 2024. PMID: 38338412 Free PMC article.
-
Theoretical study on the radical scavenging activity of gallic acid.Heliyon. 2023 Jan 5;9(1):e12806. doi: 10.1016/j.heliyon.2023.e12806. eCollection 2023 Jan. Heliyon. 2023. PMID: 36691549 Free PMC article.
-
Modulation of COX-2 and NADPH oxidase-4 by alpha-lipoic acid ameliorates busulfan-induced pulmonary injury in rats.Heliyon. 2021 Oct 13;7(10):e08171. doi: 10.1016/j.heliyon.2021.e08171. eCollection 2021 Oct. Heliyon. 2021. PMID: 34746462 Free PMC article.
-
Comparative Study of Antioxidant Potential of Selected Dietary Vitamins; Computational Insights.Molecules. 2019 Apr 26;24(9):1646. doi: 10.3390/molecules24091646. Molecules. 2019. PMID: 31027343 Free PMC article.
References
-
- Lodge JK, Youn HD, Handelman GJ, Konishi T, Matsugo S, Mathur VV, Packer L. Natural sources of lipoic acid: determination of lipoyllysine released from protease-digested tissues by high performance liquid chromatography incorporating electrochemical detection. J Appl Nutr. 1997;49:3–11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

