C6-C8 bridged epothilones: consequences of installing a conformational lock at the edge of the macrocycle
- PMID: 22127984
- PMCID: PMC3248799
- DOI: 10.1002/chem.201102630
C6-C8 bridged epothilones: consequences of installing a conformational lock at the edge of the macrocycle
Abstract
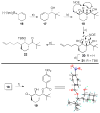
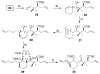
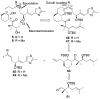
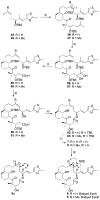
A series of conformationally restrained epothilone analogues with a short bridge between the methyl groups at C6 and C8 was designed to mimic the binding pose assigned to our recently reported EpoA-microtubule binding model. A versatile synthetic route to these bridged epothilone analogues has been successfully devised and implemented. Biological evaluation of the compounds against A2780 human ovarian cancer and PC3 prostate cancer cell lines suggested that the introduction of a bridge between C6-C8 reduced potency by 25-1000 fold in comparison with natural epothilone D. Tubulin assembly measurements indicate these bridged epothilone analogues to be mildly active, but without significant microtubule stabilization capacity. Molecular mechanics and DFT energy evaluations suggest the mild activity of the bridged epo-analogues may be due to internal conformational strain.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
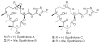
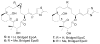
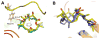
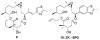
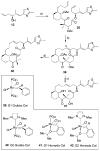
 ), 5a (
), 5a (
 ), 5 (
), 5 (
 ), 6 (
), 6 (
 ), 7 (
), 7 (
 ), 8 (
), 8 (
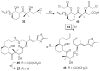
 ), 36 (
), 36 (
 ), 37 (
), 37 (
 ), 38 (
), 38 (
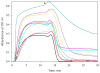
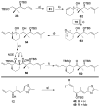
 ). Tubulin (10 μM) was incubated with 50 μM of a bridged epothilone or 10 μM PTX in the presence of 1 mM GTP and 4% DMSO in PME. Assembly was monitored in terms of apparent absorption at 350 nm. The arrow indicates temperature drop from 37 °C to 4 °C. For reference, a tubulin sample in the absence of promoter (–) was also included.
). Tubulin (10 μM) was incubated with 50 μM of a bridged epothilone or 10 μM PTX in the presence of 1 mM GTP and 4% DMSO in PME. Assembly was monitored in terms of apparent absorption at 350 nm. The arrow indicates temperature drop from 37 °C to 4 °C. For reference, a tubulin sample in the absence of promoter (–) was also included.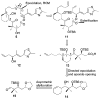


References
-
- Gerth K, Bedorf N, Hofle G, Irschik H, Reichenbach H. J Antibiot. 1996;49:560. - PubMed
-
-
For recent general reviews on epothilones, see: Altmann KH, Florsheimer A, Bold G, Caravatti G, Wartmann M. Chimia. 2004;58:686.Altmann KH. Cur Pharm Design. 2005;11:1595.Altmann KH, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. Chemmedchem. 2007;2:396.Feyen F, Cachoux F, Gertsch J, Wartmann M, Altmann K. Acc Chem Res. 2008;41:21.
-
-
- Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JT, Fojo T, Poruchynsky MS. J Biol Chem. 1997;272:17118. - PubMed
- Wolff A, Technau A, Brandner G. Int J Oncol. 1997;11:123. - PubMed
- Altmann KH, Wartmann M, O’Reilly T. Biochim Biophys Acta. 2000;1470:M79. - PubMed
- Wartmann M, Altmann KH. Curr Med Chem Anticancer Agents. 2002;2:123. - PubMed
-
- Princen K, Hatse S, Vermeire K, Aquaro S, De Clercq E, Gerlach LO, Rosenkilde M, Schwartz TW, Skerlj R, Bridger G, Schols D. J Virol. 2004;78:12996. - PMC - PubMed
- Hatse S, Princen K, De Clercq E, Rosenkilde EMM, Schwartz TW, Hernandez-Abad PE, Skerlj RT, Bridger GJ, Schols D. Biochem Pharmacol. 2005;70:752. - PubMed
- Hu JS, Freeman CM, Stolberg VR, Chiu BC, Bridger GJ, Fricker SP, Lukacs NW, Chensue SW. Am J Pathol. 2006;169:424. - PMC - PubMed
- Schwarz MK, Wells TN. Nat Rev Drug Discov. 2002;1:347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

