Heterogeneous nuclear ribonucleoprotein (hnRNP) F is a novel component of oligodendroglial RNA transport granules contributing to regulation of myelin basic protein (MBP) synthesis
- PMID: 22128153
- PMCID: PMC3265857
- DOI: 10.1074/jbc.M111.235010
Heterogeneous nuclear ribonucleoprotein (hnRNP) F is a novel component of oligodendroglial RNA transport granules contributing to regulation of myelin basic protein (MBP) synthesis
Abstract
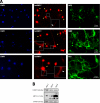
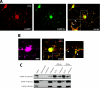
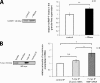
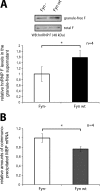
Myelin basic protein (MBP) is a major component of central nervous system (CNS) myelin. The absence of MBP results in the loss of almost all compact myelin in the CNS. MBP mRNA is sorted into RNA granules that are transported to the periphery of oligodendrocytes in a translationally inactive state. A central mediator of this transport process is the trans-acting factor heterogeneous nuclear ribonucleoprotein (hnRNP) A2 that binds to the cis-acting A2-response element in the 3'UTR of MBP mRNA. Recently, we found that activation of the Src family nonreceptor tyrosine kinase Fyn in oligodendrocytes leads to phosphorylation of hnRNP A2 and to increased translation of MBP mRNA. Here, we identify the RNA-binding protein hnRNP F as a novel component of MBP mRNA transport granules. It is associated with hnRNP A2 and MBP mRNA in cytoplasmic granular structures and is involved in post-transcriptional regulation of MBP expression. Fyn kinase activity results in phosphorylation of hnRNP F in the cytoplasm and its release from MBP mRNA and RNA granules. Our results define hnRNP F as a regulatory element of MBP expression in oligodendrocytes and imply an important function of hnRNP F in the control of myelin synthesis.
Figures




Similar articles
-
Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules.J Cell Biol. 2008 May 19;181(4):579-86. doi: 10.1083/jcb.200706164. J Cell Biol. 2008. PMID: 18490510 Free PMC article.
-
Transport and translation of MBP mRNA is regulated differently by distinct hnRNP proteins.J Cell Sci. 2014 Apr 1;127(Pt 7):1550-64. doi: 10.1242/jcs.140855. Epub 2014 Feb 12. J Cell Sci. 2014. PMID: 24522184
-
Increased expression of the MBP mRNA binding protein HnRNP A2 during oligodendrocyte differentiation.J Neurosci Res. 2004 Mar 1;75(5):614-23. doi: 10.1002/jnr.20014. J Neurosci Res. 2004. PMID: 14991837
-
RNA trafficking in oligodendrocytes.Results Probl Cell Differ. 2001;34:69-81. doi: 10.1007/978-3-540-40025-7_5. Results Probl Cell Differ. 2001. PMID: 11288680 Review.
-
Making myelin basic protein -from mRNA transport to localized translation.Front Cell Neurosci. 2013 Sep 27;7:169. doi: 10.3389/fncel.2013.00169. Front Cell Neurosci. 2013. PMID: 24098271 Free PMC article. Review.
Cited by
-
Ciphers and Executioners: How 3'-Untranslated Regions Determine the Fate of Messenger RNAs.Front Genet. 2019 Jan 24;10:6. doi: 10.3389/fgene.2019.00006. eCollection 2019. Front Genet. 2019. PMID: 30740123 Free PMC article. Review.
-
Mechanistic Target of Rapamycin Regulates the Oligodendrocyte Cytoskeleton during Myelination.J Neurosci. 2020 Apr 8;40(15):2993-3007. doi: 10.1523/JNEUROSCI.1434-18.2020. Epub 2020 Mar 5. J Neurosci. 2020. PMID: 32139584 Free PMC article.
-
Intracellular Protein Shuttling: A Mechanism Relevant for Myelin Repair in Multiple Sclerosis?Int J Mol Sci. 2015 Jul 3;16(7):15057-85. doi: 10.3390/ijms160715057. Int J Mol Sci. 2015. PMID: 26151843 Free PMC article. Review.
-
Cyclin dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation.Dev Biol. 2013 Jun 15;378(2):94-106. doi: 10.1016/j.ydbio.2013.03.023. Epub 2013 Apr 10. Dev Biol. 2013. PMID: 23583582 Free PMC article.
-
Identification and dynamic changes of RNAs isolated from RALY-containing ribonucleoprotein complexes.Nucleic Acids Res. 2017 Jun 20;45(11):6775-6792. doi: 10.1093/nar/gkx235. Nucleic Acids Res. 2017. PMID: 28379492 Free PMC article.
References
-
- Nave K. A. (2010) Myelination and the trophic support of long axons. Nat. Rev. Neurosci 11, 275–283 - PubMed
-
- Aggarwal S., Yurlova L., Simons M. (2011) Central nervous system myelin. Structure, synthesis, and assembly. Trends Cell Biol. 21, 585–593 - PubMed
-
- Emery B. (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330, 779–782 - PubMed
-
- Simons M., Trotter J. (2007) Wrapping it up. The cell biology of myelination. Curr. Opin. Neurobiol. 17, 533–540 - PubMed
-
- Griffiths I., Klugmann M., Anderson T., Yool D., Thomson C., Schwab M. H., Schneider A., Zimmermann F., McCulloch M., Nadon N., Nave K. A. (1998) Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

