Identification of a key motif that determines the differential surface levels of RET and TrkB tyrosine kinase receptors and controls depolarization enhanced RET surface insertion
- PMID: 22128160
- PMCID: PMC3265874
- DOI: 10.1074/jbc.M111.283457
Identification of a key motif that determines the differential surface levels of RET and TrkB tyrosine kinase receptors and controls depolarization enhanced RET surface insertion
Abstract
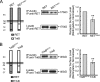
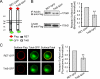
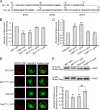
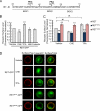
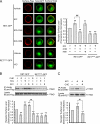
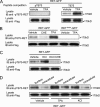
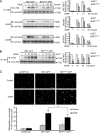
The RET tyrosine kinase receptor plays an important role in the development and maintenance of the nervous system. Although the ligand-induced RET signaling pathway has been well described, little is known about the regulation of RET surface expression, which is integral to the cell ability to control the response to ligand stimuli. We found that in dorsal root ganglion (DRG) neurons, which co-express RET and TrkB, the receptor surface levels of RET are significantly higher than that of TrkB. Using a sequence substitution strategy, we identified a key motif (Box1), which is necessary and sufficient for the differential RET and TrkB surface levels. Furthermore, pharmacological and mutagenesis assays revealed that protein kinase C (PKC) and high K(+) depolarization increase RET surface levels through phosphorylation of the Thr(675) residue in the Box1 motif. Finally, we found that the phosphorylation status of the Thr(675) residue influences RET mediated response to GDNF stimulation. In all, these findings provide a novel mechanism for the modulation of RET surface expression.
Figures



Similar articles
-
Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats.Brain Res Mol Brain Res. 2003 Jan 31;110(1):52-62. doi: 10.1016/s0169-328x(02)00584-3. Brain Res Mol Brain Res. 2003. PMID: 12573533
-
Protein-tyrosine phosphatase SHP2 contributes to GDNF neurotrophic activity through direct binding to phospho-Tyr687 in the RET receptor tyrosine kinase.J Biol Chem. 2010 Oct 8;285(41):31867-75. doi: 10.1074/jbc.M110.144923. Epub 2010 Aug 3. J Biol Chem. 2010. PMID: 20682772 Free PMC article.
-
GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia.J Comp Neurol. 2001 Nov 12;440(2):204-17. doi: 10.1002/cne.1380. J Comp Neurol. 2001. PMID: 11745618
-
Intracellular RET signaling pathways activated by GDNF.Cell Tissue Res. 2020 Oct;382(1):113-123. doi: 10.1007/s00441-020-03262-1. Epub 2020 Aug 20. Cell Tissue Res. 2020. PMID: 32816064 Review.
-
GDNF/RET signaling in dopamine neurons in vivo.Cell Tissue Res. 2020 Oct;382(1):135-146. doi: 10.1007/s00441-020-03268-9. Epub 2020 Sep 1. Cell Tissue Res. 2020. PMID: 32870383 Review.
Cited by
-
Alternative splicing results in RET isoforms with distinct trafficking properties.Mol Biol Cell. 2012 Oct;23(19):3838-50. doi: 10.1091/mbc.E12-02-0114. Epub 2012 Aug 8. Mol Biol Cell. 2012. PMID: 22875993 Free PMC article.
-
Optogenetic delivery of trophic signals in a genetic model of Parkinson's disease.PLoS Genet. 2021 Apr 15;17(4):e1009479. doi: 10.1371/journal.pgen.1009479. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33857132 Free PMC article.
-
RET overexpression leads to increased brain metastatic competency in luminal breast cancer.J Natl Cancer Inst. 2024 Oct 1;116(10):1632-1644. doi: 10.1093/jnci/djae091. J Natl Cancer Inst. 2024. PMID: 38852945 Free PMC article.
-
Super-enhancer-controlled positive feedback loop BRD4/ERα-RET-ERα promotes ERα-positive breast cancer.Nucleic Acids Res. 2022 Oct 14;50(18):10230-10248. doi: 10.1093/nar/gkac778. Nucleic Acids Res. 2022. PMID: 36124682 Free PMC article.
References
-
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. (1994) Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367, 380–383 - PubMed
-
- Baloh R. H., Enomoto H., Johnson E. M., Jr., Milbrandt J. (2000) The GDNF family ligands and receptors - implications for neural development. Curr. Opin. Neurobiol. 10, 103–110 - PubMed
-
- Airaksinen M. S., Saarma M. (2002) The GDNF family: signaling, biological functions, and therapeutic value. Nat. Rev. Neurosci. 3, 383–394 - PubMed
-
- Molliver D. C., Wright D. E., Leitner M. L., Parsadanian A. S., Doster K., Wen D., Yan Q., Snider W. D. (1997) IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19, 849–861 - PubMed
-
- Honma Y., Kawano M., Kohsaka S., Ogawa M. (2010) Axonal projections of mechanoreceptive dorsal root ganglion neurons depend on Ret. Development 137, 2319–2328 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

