Phosphatidate phosphatase plays role in zinc-mediated regulation of phospholipid synthesis in yeast
- PMID: 22128164
- PMCID: PMC3256892
- DOI: 10.1074/jbc.M111.313130
Phosphatidate phosphatase plays role in zinc-mediated regulation of phospholipid synthesis in yeast
Abstract
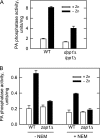
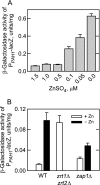
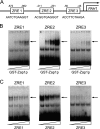
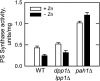
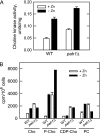
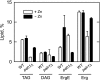
In the yeast Saccharomyces cerevisiae, the synthesis of phospholipids is coordinately regulated by mechanisms that control the homeostasis of the essential mineral zinc (Carman, G.M., and Han, G. S. (2007) Regulation of phospholipid synthesis in Saccharomyces cerevisiae by zinc depletion. Biochim. Biophys. Acta 1771, 322-330; Eide, D. J. (2009) Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 284, 18565-18569). The synthesis of phosphatidylcholine is balanced by the repression of CDP-diacylglycerol pathway enzymes and the induction of Kennedy pathway enzymes. PAH1-encoded phosphatidate phosphatase catalyzes the penultimate step in triacylglycerol synthesis, and the diacylglycerol generated in the reaction may also be used for phosphatidylcholine synthesis via the Kennedy pathway. In this work, we showed that the expression of PAH1-encoded phosphatidate phosphatase was induced by zinc deficiency through a mechanism that involved interaction of the Zap1p zinc-responsive transcription factor with putative upstream activating sequence zinc-responsive elements in the PAH1 promoter. The pah1Δ mutation resulted in the derepression of the CHO1-encoded phosphatidylserine synthase (CDP-diacylglycerol pathway enzyme) and loss of the zinc-mediated regulation of the enzyme. Loss of phosphatidate phosphatase also resulted in the derepression of the CKI1-encoded choline kinase (Kennedy pathway enzyme) but decreased the synthesis of phosphatidylcholine when cells were deficient of zinc. This result confirmed the role phosphatidate phosphatase plays in phosphatidylcholine synthesis via the Kennedy pathway.
Figures

Similar articles
-
Yeast PAH1-encoded phosphatidate phosphatase controls the expression of CHO1-encoded phosphatidylserine synthase for membrane phospholipid synthesis.J Biol Chem. 2017 Aug 11;292(32):13230-13242. doi: 10.1074/jbc.M117.801720. Epub 2017 Jul 3. J Biol Chem. 2017. PMID: 28673963 Free PMC article.
-
Phosphatidate phosphatase regulates membrane phospholipid synthesis via phosphatidylserine synthase.Adv Biol Regul. 2018 Jan;67:49-58. doi: 10.1016/j.jbior.2017.08.001. Epub 2017 Aug 16. Adv Biol Regul. 2018. PMID: 28827025 Free PMC article. Review.
-
Regulation of phospholipid synthesis in the yeast cki1Delta eki1Delta mutant defective in the Kennedy pathway. The Cho1-encoded phosphatidylserine synthase is regulated by mRNA stability.J Biol Chem. 2004 Mar 26;279(13):12081-7. doi: 10.1074/jbc.M400297200. Epub 2004 Jan 21. J Biol Chem. 2004. PMID: 14739287
-
Regulation of the Saccharomyces cerevisiae CKI1-encoded choline kinase by zinc depletion.J Biol Chem. 2008 Apr 11;283(15):10079-88. doi: 10.1074/jbc.M800502200. Epub 2008 Feb 14. J Biol Chem. 2008. PMID: 18276583 Free PMC article.
-
Regulation of phospholipid synthesis in Saccharomyces cerevisiae by zinc depletion.Biochim Biophys Acta. 2007 Mar;1771(3):322-30. doi: 10.1016/j.bbalip.2006.05.006. Epub 2006 May 19. Biochim Biophys Acta. 2007. PMID: 16807089 Free PMC article. Review.
Cited by
-
Peroxiredoxin chaperone activity is critical for protein homeostasis in zinc-deficient yeast.J Biol Chem. 2013 Oct 25;288(43):31313-27. doi: 10.1074/jbc.M113.512384. Epub 2013 Sep 10. J Biol Chem. 2013. PMID: 24022485 Free PMC article.
-
Activation of the Yeast UBI4 Polyubiquitin Gene by Zap1 Transcription Factor via an Intragenic Promoter Is Critical for Zinc-deficient Growth.J Biol Chem. 2016 Sep 2;291(36):18880-96. doi: 10.1074/jbc.M116.743120. Epub 2016 Jul 18. J Biol Chem. 2016. PMID: 27432887 Free PMC article.
-
The cellular economy of the Saccharomyces cerevisiae zinc proteome.Metallomics. 2018 Dec 12;10(12):1755-1776. doi: 10.1039/c8mt00269j. Metallomics. 2018. PMID: 30358795 Free PMC article.
-
An ER protein functionally couples neutral lipid metabolism on lipid droplets to membrane lipid synthesis in the ER.Cell Rep. 2014 Jan 16;6(1):44-55. doi: 10.1016/j.celrep.2013.11.046. Epub 2013 Dec 27. Cell Rep. 2014. PMID: 24373967 Free PMC article.
-
Pho85p-Pho80p phosphorylation of yeast Pah1p phosphatidate phosphatase regulates its activity, location, abundance, and function in lipid metabolism.J Biol Chem. 2012 Mar 30;287(14):11290-301. doi: 10.1074/jbc.M112.346023. Epub 2012 Feb 9. J Biol Chem. 2012. PMID: 22334681 Free PMC article.
References
-
- Iwanyshyn W. M., Han G. S., Carman G. M. (2004) Regulation of phospholipid synthesis in Saccharomyces cerevisiae by zinc. J. Biol. Chem. 279, 21976–21983 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

