Amer2 protein is a novel negative regulator of Wnt/β-catenin signaling involved in neuroectodermal patterning
- PMID: 22128170
- PMCID: PMC3265856
- DOI: 10.1074/jbc.M111.308650
Amer2 protein is a novel negative regulator of Wnt/β-catenin signaling involved in neuroectodermal patterning
Abstract
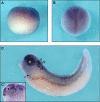
Wnt/β-catenin signaling is negatively controlled by the adenomatous polyposis coli (APC) tumor suppressor, which induces proteasomal degradation of β-catenin as part of the β-catenin destruction complex. Amer2 (APC membrane recruitment 2; FAM123A) is a direct interaction partner of APC, related to the tumor suppressor Amer1/WTX, but its function in Wnt signaling is not known. Here, we show that Amer2 recruits APC to the plasma membrane by binding to phosphatidylinositol 4,5-bisphosphate lipids via lysine-rich motifs and that APC links β-catenin and the destruction complex components axin and conductin to Amer2. Knockdown of Amer2 increased Wnt target gene expression and reporter activity in cell lines, and overexpression reduced reporter activity, which required membrane association of Amer2. In Xenopus embryos, Amer2 is expressed mainly in the dorsal neuroectoderm and neural tissues. Down-regulation of Amer2 by specific morpholino oligonucleotides altered neuroectodermal patterning, which could be rescued by expression of a dominant-negative mutant of Lef1 that interferes with β-catenin-dependent transcription. Our data characterize Amer2 for the first time as a negative regulator of Wnt signaling both in cell lines and in vivo and define Amer proteins as a novel family of Wnt pathway regulators.
Figures


Similar articles
-
Amer2 protein interacts with EB1 protein and adenomatous polyposis coli (APC) and controls microtubule stability and cell migration.J Biol Chem. 2012 Oct 12;287(42):35333-35340. doi: 10.1074/jbc.M112.385393. Epub 2012 Aug 16. J Biol Chem. 2012. PMID: 22898821 Free PMC article.
-
Adenomatous polyposis coli (APC) membrane recruitment 3, a member of the APC membrane recruitment family of APC-binding proteins, is a positive regulator of Wnt-β-catenin signalling.FEBS J. 2014 Feb;281(3):787-801. doi: 10.1111/febs.12624. Epub 2013 Dec 12. FEBS J. 2014. PMID: 24251807
-
Structural and functional characterization of the Wnt inhibitor APC membrane recruitment 1 (Amer1).J Biol Chem. 2011 Jun 3;286(22):19204-14. doi: 10.1074/jbc.M111.224881. Epub 2011 Apr 15. J Biol Chem. 2011. PMID: 21498506 Free PMC article.
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
-
The Yin-Yang of TCF/beta-catenin signaling.Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review.
Cited by
-
A multi-omics approach to visualize early neuronal differentiation from hESCs in 4D.iScience. 2022 Oct 4;25(11):105279. doi: 10.1016/j.isci.2022.105279. eCollection 2022 Nov 18. iScience. 2022. PMID: 36304110 Free PMC article.
-
Loss of Peter Pan (PPAN) Affects Mitochondrial Homeostasis and Autophagic Flux.Cells. 2019 Aug 14;8(8):894. doi: 10.3390/cells8080894. Cells. 2019. PMID: 31416196 Free PMC article.
-
Structures of the APC-ARM domain in complexes with discrete Amer1/WTX fragments reveal that it uses a consensus mode to recognize its binding partners.Cell Discov. 2015 Jul 14;1:15016. doi: 10.1038/celldisc.2015.16. eCollection 2015. Cell Discov. 2015. PMID: 27462415 Free PMC article.
-
Amer2 protein interacts with EB1 protein and adenomatous polyposis coli (APC) and controls microtubule stability and cell migration.J Biol Chem. 2012 Oct 12;287(42):35333-35340. doi: 10.1074/jbc.M112.385393. Epub 2012 Aug 16. J Biol Chem. 2012. PMID: 22898821 Free PMC article.
-
Druggable upregulated proteins in EWS-FLI-driven Ewing sarcoma as emerging new therapeutic targets.Am J Transl Res. 2025 Mar 15;17(3):1580-1603. doi: 10.62347/YMEU1808. eCollection 2025. Am J Transl Res. 2025. PMID: 40225989 Free PMC article. Review.
References
-
- Polakis P. (2000) Wnt signaling and cancer. Genes Dev. 14, 1837–1851 - PubMed
-
- Grohmann A., Tanneberger K., Alzner A., Schneikert J., Behrens J. (2007) AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J. Cell Sci. 120, 3738–3747 - PubMed
-
- Major M. B., Camp N. D., Berndt J. D., Yi X., Goldenberg S. J., Hubbert C., Biechele T. L., Gingras A. C., Zheng N., Maccoss M. J., Angers S., Moon R. T. (2007) Wilms tumor suppressor WTX negatively regulates WNT/β-catenin signaling. Science 316, 1043–1046 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

