The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore
- PMID: 22128171
- PMCID: PMC3268414
- DOI: 10.1074/jbc.M111.275354
The C terminus of Bax inhibitor-1 forms a Ca2+-permeable channel pore
Abstract
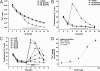
Bax inhibitor-1 (BI-1) is a multitransmembrane domain-spanning endoplasmic reticulum (ER)-located protein that is evolutionarily conserved and protects against apoptosis and ER stress. Furthermore, BI-1 is proposed to modulate ER Ca(2+) homeostasis by acting as a Ca(2+)-leak channel. Based on experimental determination of the BI-1 topology, we propose that its C terminus forms a Ca(2+) pore responsible for its Ca(2+)-leak properties. We utilized a set of C-terminal peptides to screen for Ca(2+) leak activity in unidirectional (45)Ca(2+)-flux experiments and identified an α-helical 20-amino acid peptide causing Ca(2+) leak from the ER. The Ca(2+) leak was independent of endogenous ER Ca(2+)-release channels or other Ca(2+)-leak mechanisms, namely translocons and presenilins. The Ca(2+)-permeating property of the peptide was confirmed in lipid-bilayer experiments. Using mutant peptides, we identified critical residues responsible for the Ca(2+)-leak properties of this BI-1 peptide, including a series of critical negatively charged aspartate residues. Using peptides corresponding to the equivalent BI-1 domain from various organisms, we found that the Ca(2+)-leak properties were conserved among animal, but not plant and yeast orthologs. By mutating one of the critical aspartate residues in the proposed Ca(2+)-channel pore in full-length BI-1, we found that Asp-213 was essential for BI-1-dependent ER Ca(2+) leak. Thus, we elucidated residues critically important for BI-1-mediated Ca(2+) leak and its potential channel pore. Remarkably, one of these residues was not conserved among plant and yeast BI-1 orthologs, indicating that the ER Ca(2+)-leak properties of BI-1 are an added function during evolution.
Figures





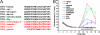

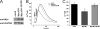
Similar articles
-
Bax Inhibitor-1-mediated Ca2+ leak is decreased by cytosolic acidosis.Cell Calcium. 2013 Sep;54(3):186-92. doi: 10.1016/j.ceca.2013.06.002. Epub 2013 Jul 16. Cell Calcium. 2013. PMID: 23867001
-
Bax inhibitor-1 is likely a pH-sensitive calcium leak channel, not a H+/Ca2+ exchanger.Sci Signal. 2014 Sep 16;7(343):pe22. doi: 10.1126/scisignal.2005764. Sci Signal. 2014. PMID: 25227609
-
Live cell calcium imaging combined with siRNA mediated gene silencing identifies Ca²⁺ leak channels in the ER membrane and their regulatory mechanisms.J Vis Exp. 2011 Jul 7;(53):e2730. doi: 10.3791/2730. J Vis Exp. 2011. PMID: 21775954 Free PMC article.
-
Presenilins as endoplasmic reticulum calcium leak channels and Alzheimer's disease pathogenesis.Sci China Life Sci. 2011 Aug;54(8):744-51. doi: 10.1007/s11427-011-4201-y. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786197 Review.
-
A comprehensive overview of the complex world of the endo- and sarcoplasmic reticulum Ca2+-leak channels.Biochim Biophys Acta Mol Cell Res. 2021 Jun;1868(7):119020. doi: 10.1016/j.bbamcr.2021.119020. Epub 2021 Mar 30. Biochim Biophys Acta Mol Cell Res. 2021. PMID: 33798602 Review.
Cited by
-
Bax inhibitor 1 is a γ-secretase-independent presenilin-binding protein.Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):141-147. doi: 10.1073/pnas.1810870116. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559186 Free PMC article.
-
The selective BH4-domain biology of Bcl-2-family members: IP3Rs and beyond.Cell Mol Life Sci. 2013 Apr;70(7):1171-83. doi: 10.1007/s00018-012-1118-y. Epub 2012 Sep 6. Cell Mol Life Sci. 2013. PMID: 22955373 Free PMC article. Review.
-
TMBIM5 loss of function alters mitochondrial matrix ion homeostasis and causes a skeletal myopathy.Life Sci Alliance. 2022 Jun 17;5(10):e202201478. doi: 10.26508/lsa.202201478. Print 2022 Oct. Life Sci Alliance. 2022. PMID: 35715207 Free PMC article.
-
Bioinformatic analyses of integral membrane transport proteins encoded within the genome of the planctomycetes species, Rhodopirellula baltica.Biochim Biophys Acta. 2014 Jan;1838(1 Pt B):193-215. doi: 10.1016/j.bbamem.2013.08.007. Epub 2013 Aug 19. Biochim Biophys Acta. 2014. PMID: 23969110 Free PMC article.
-
ITPRs/inositol 1,4,5-trisphosphate receptors in autophagy: From enemy to ally.Autophagy. 2015;11(10):1944-8. doi: 10.1080/15548627.2015.1083666. Autophagy. 2015. PMID: 26291777 Free PMC article. Review.
References
-
- Sammels E., Parys J. B., Missiaen L., De Smedt H., Bultynck G. (2010) Intracellular Ca2+ storage in health and disease. A dynamic equilibrium. Cell Calcium 47, 297–314 - PubMed
-
- Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signaling. Dynamics, homeostasis, and remodeling. Nat. Rev. Mol. Cell Biol. 4, 517–529 - PubMed
-
- Madeo F., Kroemer G. (2009) Intricate links between ER stress and apoptosis. Mol. Cell 33, 669–670 - PubMed
-
- Austin R. C. (2009) The unfolded protein response in health and disease. Antioxid. Redox Signal. 11, 2279–2287 - PubMed
-
- D'hondt C., Ponsaerts R., De Smedt H., Vinken M., De Vuyst E., De Bock M., Wang N., Rogiers V., Leybaert L., Himpens B., Bultynck G. (2011) Pannexin channels in ATP release and beyond. An unexpected rendezvous at the endoplasmic reticulum. Cell Signal. 23, 305–316 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

