Insulin-like growth factor-I receptor (IGF-IR) translocates to nucleus and autoregulates IGF-IR gene expression in breast cancer cells
- PMID: 22128190
- PMCID: PMC3268434
- DOI: 10.1074/jbc.M111.281782
Insulin-like growth factor-I receptor (IGF-IR) translocates to nucleus and autoregulates IGF-IR gene expression in breast cancer cells
Abstract
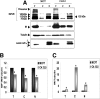
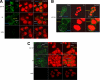
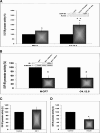
The insulin-like growth factor (IGF) system plays an important role in mammary gland biology as well as in the etiology of breast cancer. The IGF-I receptor (IGF-IR), which mediates the biological actions of IGF-I and IGF-II, has emerged in recent years as a promising therapeutic target. The IGF and estrogen signaling pathways act in a synergistic manner in breast epithelial cells. The present study was aimed at investigating 1) the putative translocation of IGF-IR and the related insulin receptor (IR) to the nucleus in breast cancer cells, 2) the impact of IGF-IR and IR levels on IGF-IR biosynthesis in estrogen receptor (ER)-positive and ER-depleted breast cancer cells, and 3) the potential transcription factor role of IGF-IR in the specific context of IGF-IR gene regulation. We describe here a novel mechanism of autoregulation of IGF-IR gene expression by cellular IGF-IR, which is seemingly dependent on ER status. Regulation of the IGF-IR gene by IGF-IR protein is mediated at the level of transcription, as demonstrated by 1) binding assays (DNA affinity chromatography and ChIP) showing specific IGF-IR binding to IGF-IR promoter DNA and 2) transient transfection assays showing transactivation of the IGF-IR promoter by exogenous IGF-IR. The IR is also capable of translocating to the nucleus and binding the IGF-IR promoter in ER-depleted, but not in ER-positive, cells. However, transcription factors IGF-IR and IR display diametrically opposite activities in the context of IGF-IR gene regulation. Thus, whereas IGF-IR stimulated IGF-IR gene expression, IR inhibited IGF-IR promoter activity. In summary, we have identified a novel mechanism of IGF-IR gene autoregulation in breast cancer cells. The clinical implications of these findings and, in particular, the impact of IGF-IR/IR nuclear localization on targeted therapy require further investigation.
Figures



References
-
- Surmacz E. (2000) Function of the IGF-I receptor in breast cancer. J. Mammary Gland Biol. Neoplasia 5, 95–105 - PubMed
-
- Chitnis M. M., Yuen J. S., Protheroe A. S., Pollak M., Macaulay V. M. (2008) The type 1 insulin-like growth factor receptor pathway. Clin. Cancer Res. 14, 6364–6370 - PubMed
-
- Werner H., Bruchim I. (2009) The insulin-like growth factor-I receptor as an oncogene. Arch. Physiol. Biochem. 115, 58–71 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

